LITERATURE - 2007, Journal of Hungarian Obstetricians and Gynaecologists
Fetal growth and insulin-like growth factor system

Author: Mitsutoshi Iwashita, MD
Department of Obstetrics and Gynecology, Kyorin University School of Medicine, Tokyo
Insulin-like growth factors (IGF-I and IGF-II) stimulate extravillous trophoblast (EVT) cells migration and invasion into uterine wall and one of IGF binding proteins (IGFBPs), IGFBP-1 inhibits IGF action thereby regulating EVT cells invasion negatively. Thus, appropriate placentation is determined by the balance of IGF and IGFBP-1 in maternal-fetal interface. IGF-I stimulates amino acids uptake by trophoblast cells in vitro and enhances the transfer of maternal amino acids to fetus in vivo. In contrast, IGFBP-1 inhibits IGF-I action in placenta in terms of maternal amino acids transfer to fetus. In mother, circulating levels of IGF-I are increased during pregnancy and correlate with birth weight while IGFBP-1 gradually increased throughout pregnancy and negatively correlates with birth weight. Thus, maternal IGF-I and IGFBP-1 are tightly involved in fetal growth presumably by regulating placental nutrient transfer to fetus. Fetal circulating levels of IGF-I are positively and IGFBP-1 are negatively correlate with birth weight as well. Cell culture and animal experiments clearly demonstrate that fetal IGF-I and IGFBP-1 are regulated by nutritional factors where fetus inhibits IGFBP-1 production under enough supply of nutrition from placenta and promotes its own growth. A condition that decreases supply of these substances such as placental dysfunction, fetus produces more IGFBP-1 and inhibits IGF-I action in order to inhibit own growth to survive. Although fetal circulating levels of IGF-I are much lower than those in mother, different profiles of phosphoisoforms of IGFBP-1 between mother and fetus may explain remarkable fetal growth due to high bioactivity of IGF-I in fetus.
Keywords: fetal growth, placenta, IGF, IGFBP-1, phosphoisoforms
IGF system
Insulin-like growth factor (IGF) is one of growth factors that has insulin like activity. There are two similar peptides, namely IGF-I and IGF-II [1, 2] that are interacting with their receptors (type I and II receptors) on cell surface. Most of biological actions of IGF is believed to be mediated through type I IGF receptor (IGF-I receptor) that structure is similar to insulin receptor. Most IGFs are bound to specific binding proteins in biological fluids and at present six distinct IGF binding proteins are identified namely IGFBP-1, 2, 3, 4, 5 and 6 [3]. IGFBPs mostly inhibit but in some case enhance IGF action [4]. IGFBPs are proteolysed by specific proteases [5, 6] that indirectly modify IGF action (Figure 1).
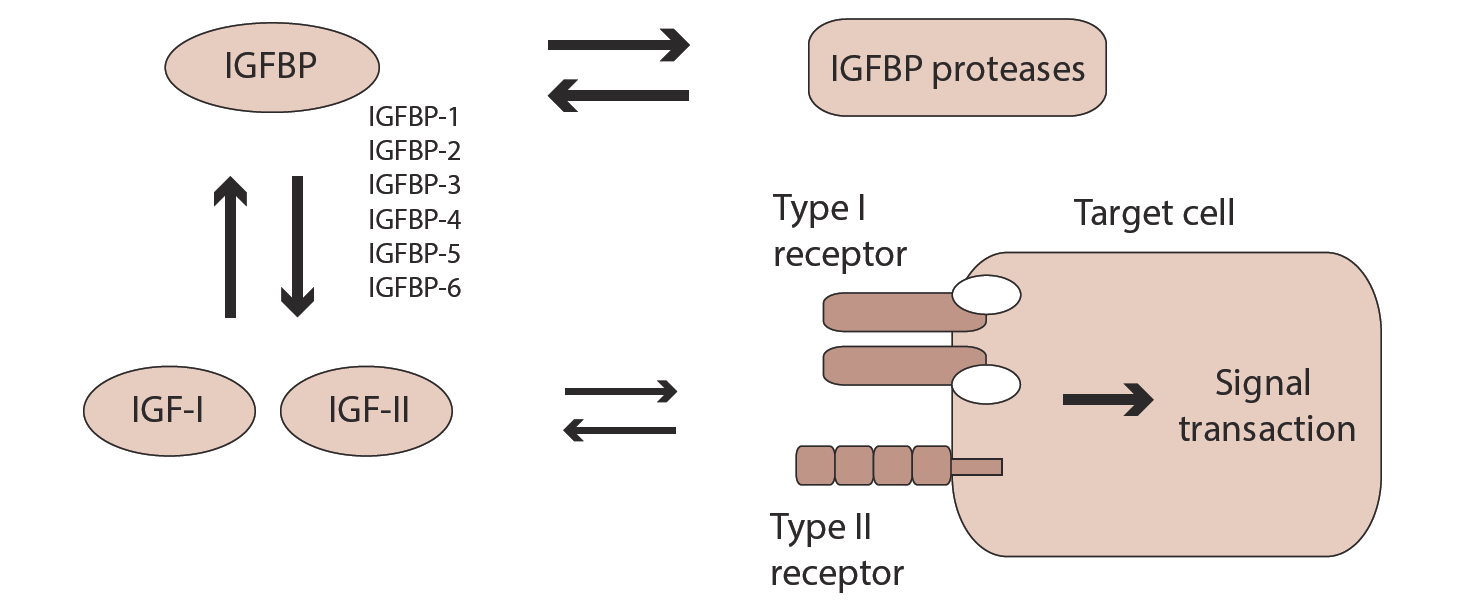
IGF action in trophoblast cells
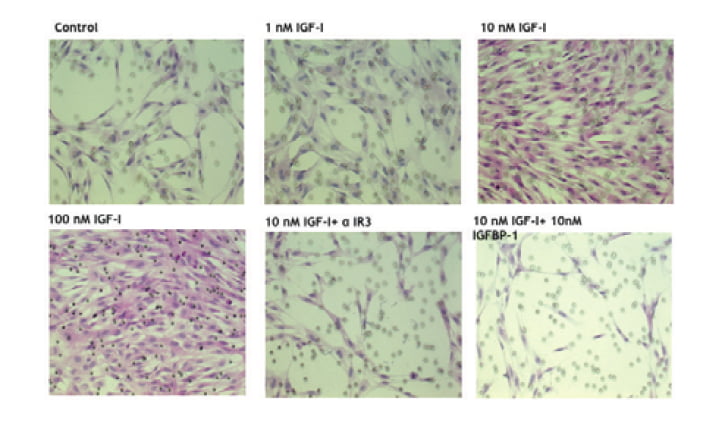
Implantation process consists of two cell biological events including attachment and invasion of trophobast cells [7]. In invasion process, trophoblast cells migrate and proteolyse extracellular matrix of uterine endometrial cells. IGF-I treatment causes remarkable changes of cell shape in which extravillous trophoblast (EVT) cells extend lamelipodia and attach strongly on fibronectin coated culture dish (Figure 2) [8]. Attachment assay and migration assay clearly demonstrate that IGF-I stimulates extravillous trophoblast attachment and migration. In attachment assay, IGF-I stimulates EVT cells attachment dose dependently (Figure 3 and 4) [8] and IGFBP-1 inhibits IGF-I action. IGF-I induced attachment is abolished by the addition of alpha IR3, an IGF-I receptor antibody (Figure 4) suggesting that IGF-I stimulates cell attachment through IGF-I receptor.
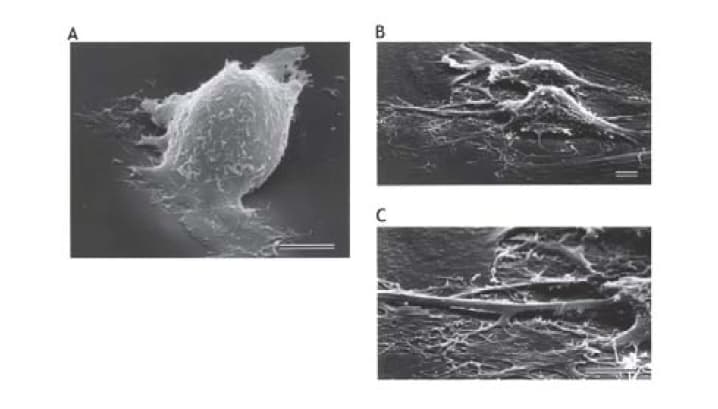
Serum-starved EVT cells are seeded and treated for 2 hr with serum-free medium containing no addition (A) or 10 nM IGF-I (B and C). Scale bars, 10 µm.
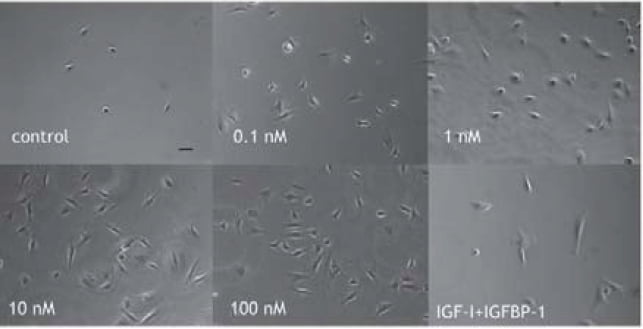
EVT cells are incubated in culture dish in the presence of IGF-I (0.1-100 nM) or 10 nM IGF-I + 10 nM IGFBP-1 for 1 hr. Then atteched cell number on dish is counted after several washes.
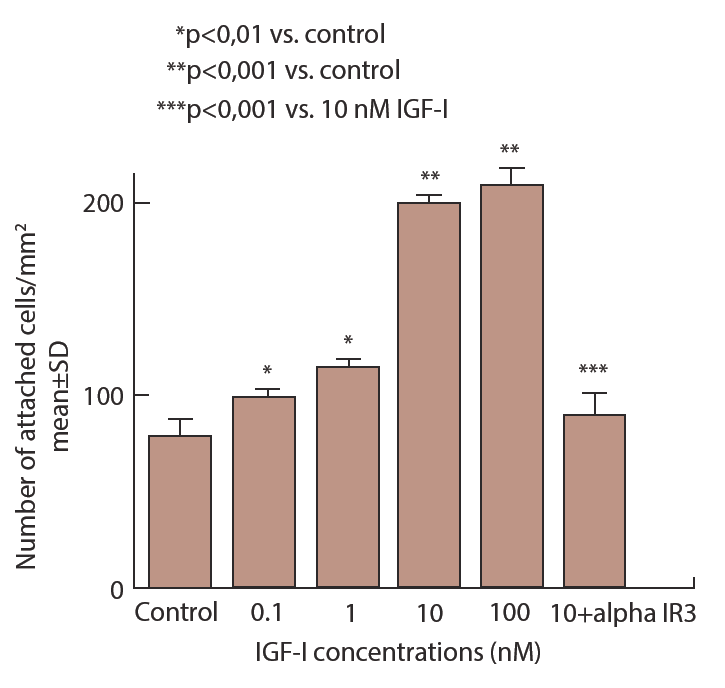
EVT cells are incubated in culture dish in the presence of IGF-I (0.1-100 nM) or 10 nM IGF-I + 10 nM alpha IRЗ for 1 hr. Then attached cell number on dish is counted after several washes.
In migration assay, EVT cells migrated through pores on the bottom of inner culture well are increased by IGF-I dose dependently [9] and this is inhibited by the addition of alpha IR3 with IGF-I suggesting that IGF-I stimulates trophoblast migration through its receptor as well (Figure 5). IGF-I-stimulated cell migration was also blocked by IGFBP-1 (Figure 5).
EVT cells are cultured in inner well of double chanber that has small pores on the bottom. Cells are incubated for 24 hr in the presence of indicated concentrations of IGF-I, 10 nМ alpha IPЗ or 10 nМ IGFBP-1 in the presence of 10nM IGF-I and cells passed through pores are stained and counted
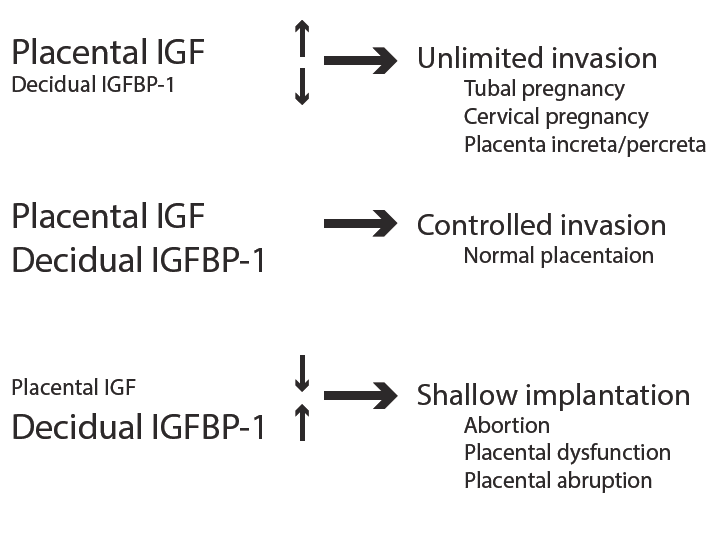
Placenta produces IGF-I and IGF-II and their receptors [10, 11] that stimulate trophoblast migration and proliferation in an autocrine fashion. In contrast, decidual cells produce large amounts of IGFBP-1 [12] that inhibits IGF action [13, 14]. Therefore, it is suggested that the balance of placental IGF and decidual IGFBP-1 production is important for controlled trophoblast invasion into uterine endometrium. If trophoblastic IGF production is exceeded than decidual IGFBP-1 production, trophoblast invades unlimitedly that is seen in tubal pregnancy, cervical pregnancy and placental increta and percreta. In contrast, over production of IGFBP-1 in decidua compared to IGF production by placenta causes so called shallow implantation that is seen in abortion, placental dysfunction and placental abruption. Thus, the imbalance of local IGF and IGFBP-1 production might be involved in pathogenesis of abnormal pregnancy (Figure 6).
IGF and fetal growth
It has been demonstrated that maternal IGF-I increased during pregnancy, especially in the third trimester [15]. IGF-I is regulated by pituitary GH, however, maternal IGF-I is believed to be regulated by placental hormones such as placental GH variant [16] rather than pituitary GH during pregnancy that is responsible for increased levels of IGF-I in the maternal circulation. Free IGF-I levels that are unbound to IGFBPs also increased in the third trimester suggesting that IGF-I bioactivity is inсrеasеd in the third trimester as well.
It is well documented that maternal levels of IGF-I are correlated with birth weight [17]. Recent studies have demonstrated that binding activities of IGFBPs in maternal circulation was remarkably reduced during pregnancy due to increased protease activity in the maternal circulation [18, 19]. When maternal IGFBPs are analyzed by ligand blot, the binding activities of IGFBP-3, IGFBP-2 and IGFBP-4 are reduced along with gestational age while binding activity of IGFBP-1 is increased throughout pregnancy (Figure 7) [20] and its level is inversely correlated with birth weight [15, 21].
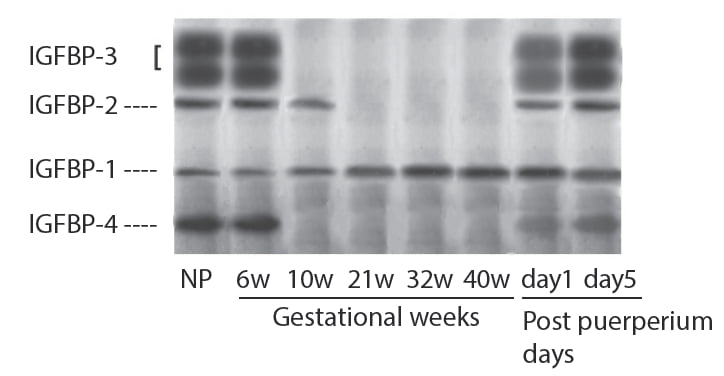
These changes of IGFBPs are quickly returned as early as day 1 of post puerperium. Thus, maternal IGF-I and IGFBP-1 seem to play important role in fetal growth and balance of both substances may determine fetal growth. Maternal IGF-I can not to be transferred to fetal circulation through the placenta and placenta is found to contain IGF-I receptor [10, 11]. Therefore, maternal IGF-I stimulates fetal growth through the placenta presumably by activating nutrients transfer to the fetus through placenta. IGF-I stimulates 3H-glycine uptake and release by cultured trophoblast cells and IGFBP-1 inhibits stimulatory effect of IGF-I dose dependently [22]. Furthermore, fetal weight is reduced in anti-IGF-I antiserum treated mice and transfer of ЗH-aminoisobutyric acid (ЗH-AIB) to fetus that is injected to maternal mice is also decreased. In contrast, fetal weight and transfer of 3H-AIB to fetus are increased in anti-IGFBP-1 antiserum treated mice (Figure 8) [22] suggesting that fetal growth and 3H-AIB transfer are accelerated by the immunoneutralization of IGFBP-1.
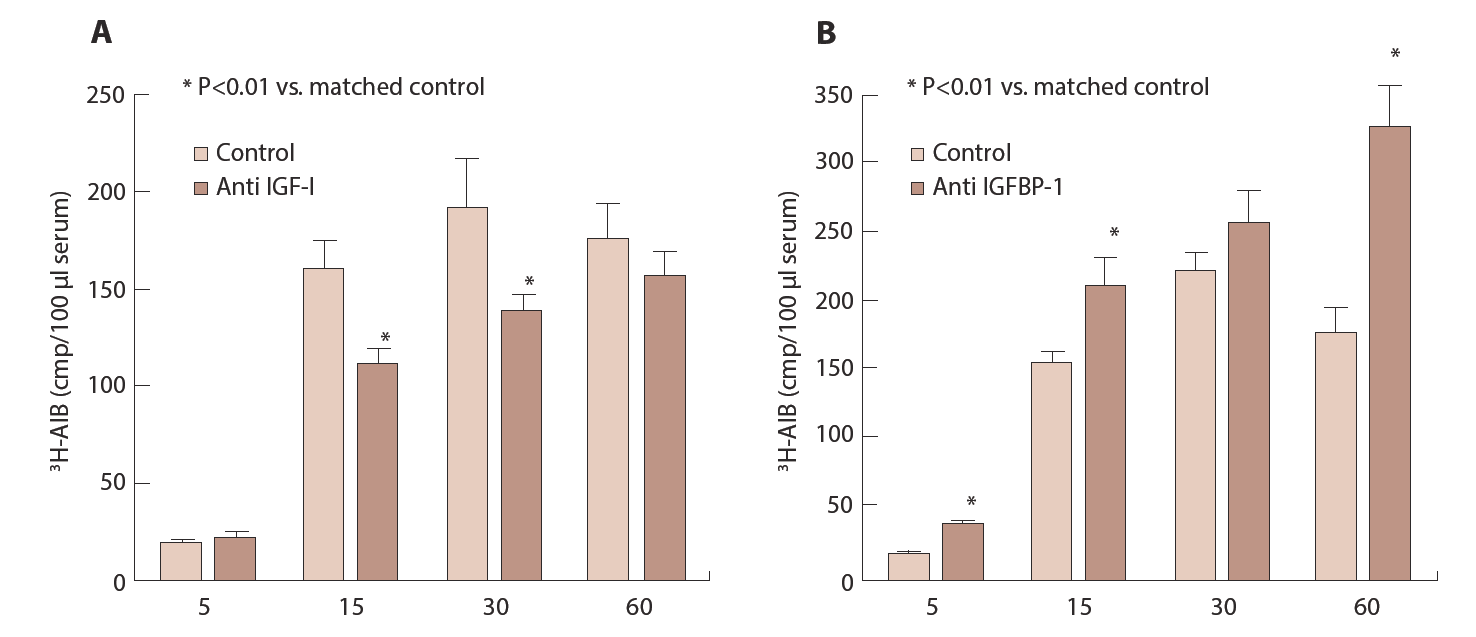
Anti IGF-I or IGFBP-1 is injected to pregnant mice between Day 14 and 17. On Day 18, 5µCi 3H-AIB is injected to maternal mice and fetuses are removed at indicated time and sera from fetuses is pooled and radioactivity is measured.
Many studies indicate that IGFBP-1 inhibits biological action of IGF-I [13, 14, 23, 24] and this inhibitory action of IGFBP-1 is reported to be achieved by inhibiting binding of IGF-I to its receptors [25, 26]. Thus, maternal IGF-I stimulates fetal growth by activating placental transport system that increases in nutrient supply from mother to fetus. In contrast, maternal IGFBP-1 inhibits fetal growth by inhibiting IGF-I access to its receptor on placenta thereby suppressing IGF action on placenta and the imbalance of maternal IGF-I and IGFBP-1 levels might be involved in pathogenesis of fetal growth restriction (FGR).
As observed in maternal circulation, fetal circulating IGF-I is positively [27] and IGFBP-1 is negatively [28, 29] correlated with birth weight. Fetal IGF-I and IGFBP-1 levels are independent from their mother and regulated by nutritional condition. Major production site of IGFBP-1 in fetus is liver and fetal rat liver cell culture system shows that IGFBP-1 in medium is increased in the absence of glucose and amino acids in the medium [30] suggesting that fetal IGFBP-1 is increased in poor nutritional condition in vitro. A part of molecular mechanism at transcriptional level by which nutritional factors regulate IGFBP-1 production is becoming clear. It is well known that insulin response element (IRE) and glucocorticoid response element (GRE) exist in promoter gene of IGFBP-1 that inhibits and stimulates IGFBP-1 production, respectively [31, 32]. In addition, it become clear that amino acid response element exists between - 112 and -81 bp from the cap site that includes IRE and GRE region [33]. Among various kind of amino acids, levels of branched chain amino acids (BCAA) in cord sera are selectively decreased in small for gestational age (SGA) infants compared to those in appropriate gestational age (AGA) infants [34]. Deprivation of BCAA stimulates IGFBP-1 production in various cell culture system [35, 36] suggesting that deficiency of BCAA in FGR fetus might be involved in pathogenesis of FGR. Since regulation of protein synthesis by BCAA is mediated by mammalian target of rapamycin (mTOR) signaling pathway [37], IGFBP-1 production might be controlled by this signaling pathway. These results suggest that fetal IGFBP-1 is regulated not only by hormones but also by nutritional factors. In vivo experiment also suggests nutritional regulation of IGFBP-1 in fetus. Rat FGR fetus by maternal starvation by which fetal weight was reduced to 65% of control shows reduced IGF-I but not IGF-II levels in the circulation. In contrast, increased mRNA for IGFBP-1 in fetal liver is observed in FGR fetus while there is no difference in IGFBP-2 mRNA between control and FGR fetus (Figure 9) suggesting that increased IGFBP-1 in FGR fetus is regulated at transcriptional level. Thus, FGR may not be passive reaction of fetus corresponding to decrease in maternal nutrients supply but may be active, self-protecting action to survive themselves.
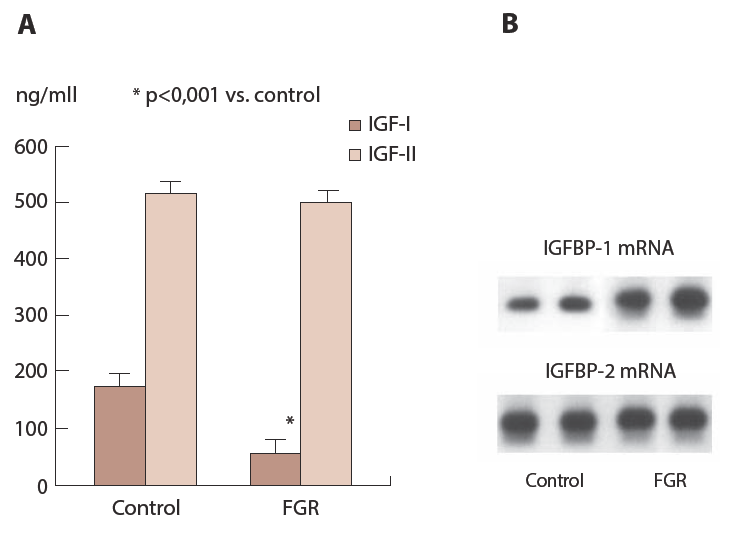
Maternal rats are starved between Day 16 and 19 and fetal blood is collected at Day 20 and measured IGF-I and IGF-II by ELISА kits (A) and mRNA for IGFBP-1 and -2 in fetal liver is analyzed by nortern blot (B).
Phosphoisoforms of IGFBP-1 in mother and fetus
Levels of IGF-I in the fetal circulation are extremely low while levels of IGFBP-1 are high compared to those of maternal circulation (Figure 10). A contradiction between fetal developmental speed and high levels of IGFBP-1 and low levels of IGF-I in fetus suggest that the mechanism in the fetus that can mediate fetal remarkable growth is different from those in maternal side. Recently phosphorylated forms of IGFBP-1 have been reported [38] in which three serine residues in the molecule can be phosphorylated [39].
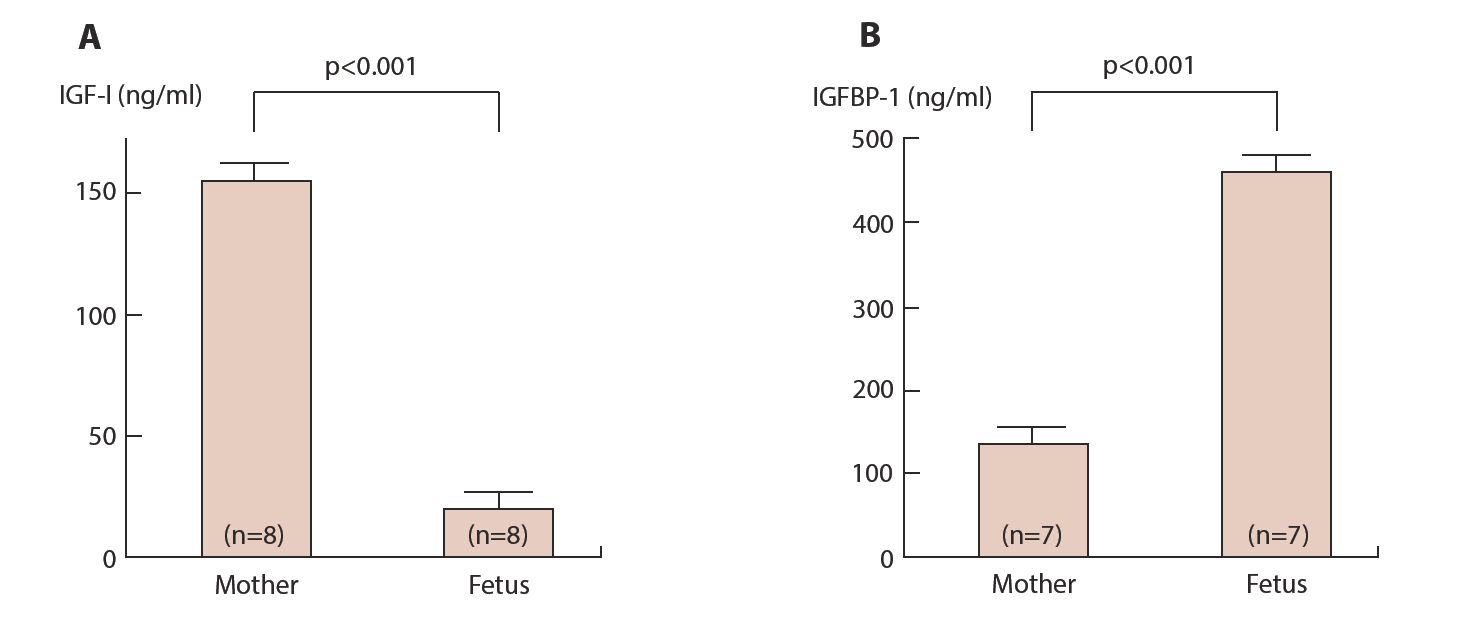
Maternal blood and cord blood from their fetuses are collected at term delivery and IGF-I (А) and IGFBP-1 (B) are measured by ELISA kits.
Although non-phosphorylated and phosphorylated forms of IGFBP-1 have identical molecular weight, these isoforms can be separated based on difference of electrical charge of each molecule by non-denaturing gel electrophoresis and anion exchange chromatography [40] and one non-phosphorylated and four to five phosphorylated IGFBP-1 are identified. Phosphorylated IGFBP-1 has higher affinity for IGF-I than non-phosphorylated IGFBP-1 [38, 39] and interestingly, IGF-I-stimulated 3Н-AIB uptake by cultured fibroblast cells derived from term placenta is inhibited by phosphorylated IGFBP-1 while non-phosphorylated IGFBP-1 enhances IGF-I action (Figure 11) [41, 42] suggesting that non-phosphorylated and phosphorylated IGFBP-1 have absolutely different biological effect on IGF action. Similar opposite effects of phosphoisoforms of IGFBP-1 on IGF-I action have been reported in vitro and in vivo [43, 44].
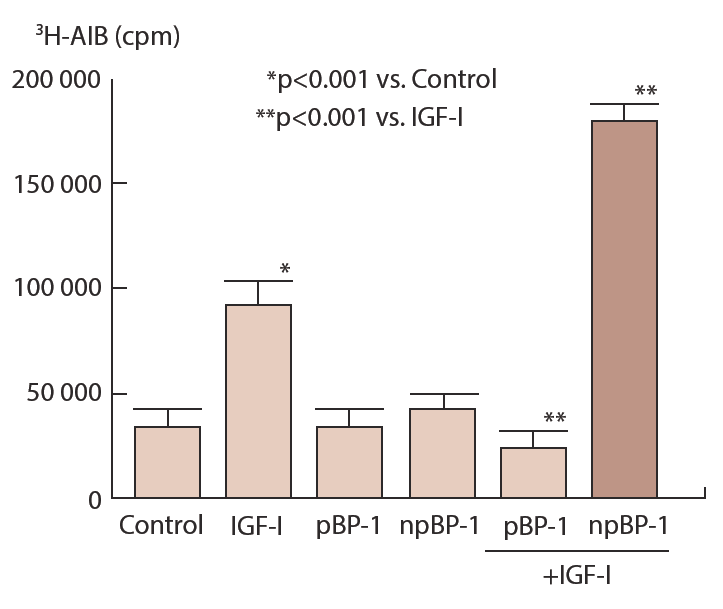
Phosphorylated (pBP-1) and nonphosphorylated (npBP-1) in pooled amniotic fluids are separated by anion exchange column of HPLC. Fibroblast cells derived from term placenta are cultured in the presence or absence of 10 nM IGFBP-1 phosphoisoforms for 24hr and further incubate with or without 10 nM IGF-I for 3 hr followed by incubation with 1µCi of 3H-AIB for 30 min. Incorporated redioactivity into cells is counted in a scintillation counter after solubilization.
Phosphoisoforms of IGFBP-1 separated by anion exchange chromatography demonstrates that the proportion of non-phosphorylated IGFBP-1 to total IGFBP-1 is significantly higher in infants than their mothers (Figure 12) although total amounts of IGFBP-1 are higher in infants than in mothers. This may suggest that biological activity of IGF is higher in fetus compared to their mothers and it may be a possible explanation for remarkable growing speed observed in fetus even in high levels of IGFBP-1.
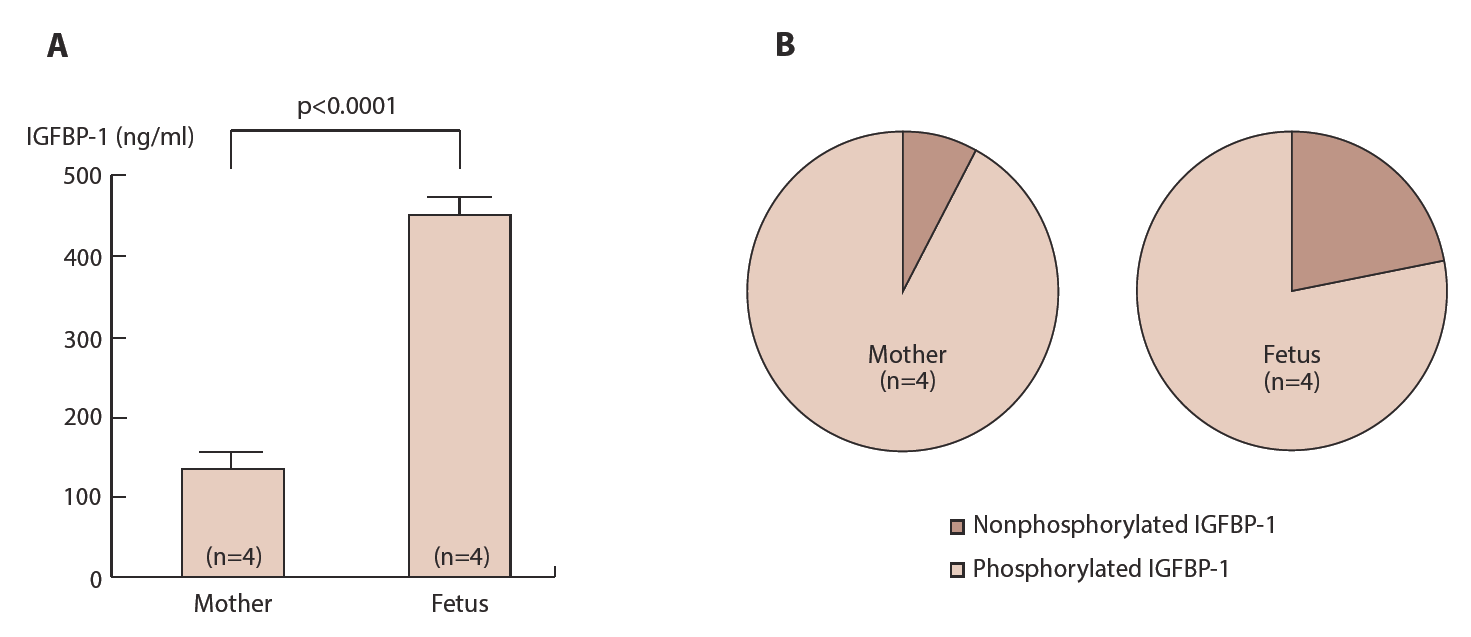
Blood smples are collected form mothers and their fetuses at delivery between 28 and 34 weeks gestation. Total IGFBP-1 is measured by ELISА (A). Phosphoisoforms of IGFBP-1 are separated by anion exchange column of HPLC and IGFBP-1 in each fractions is measured by ELISA and expresses as percent of total IGFBP-1 (B).
IGFBP-1 is phosphorylated intracellularly by various kinases in vivo and in vitro and phosphorylated IGFBP-1 is specifically increased in a catabolic state such as severe trauma or diabetes mellitus [45, 46]. Only phosphorylated forms of IGFBP-1 are increased when rat fetal liver cells are cultured in the absence of amino acids (Figure 13).
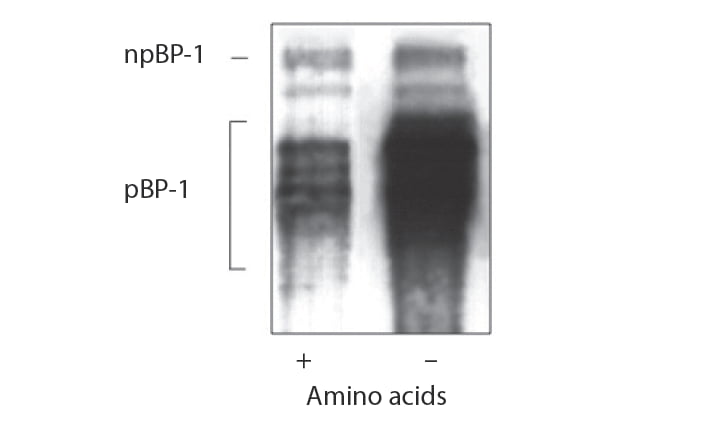
Rat fetal liver cells are cultured in the presence or absence of amino acids in the medium for 24 hr and medium is concentrated and subjected to non-denaturing polycrylamide gel electrophoresis. Nonphorylated (npBP-1) and phosporylated (pEP-1) IGFBP-1 are then analyzed by immunoblot
In human, total IGFBP-1 levels are higher in SGA fetuses than in AGA fetuses and phosphorylated IGFBP-1 was higher in SGA fetuses than in AGA fetuses although non-phosphorylated IGFBP-1 levels are similar betveen two groups (Table 1) [42, 47]. Thus, biological activity of IGF-I in SGA fetus is presumed to be more suppressed than in AGA fetus. These phenomena also support self-protecting mechanism in fetus by which fetuses restrict their growth to survive in malnutritional environments.
| Table 1. Profiles of IGFBP-1 phosphoisoforms in AGA and SGA fetuses at term | ||
|---|---|---|
| AGA (n=15) | SGА (n=10) | |
| Gestational age (wks) | 37.8±1.7 | 38.4±2.6 |
| Birth weight (g) | 3108±198 | 2317±114а |
| Total IGFBР-1 (ng/ml) | 105.5±12.3 | 255.5±25.9b |
| nplGFBP-1 | 36.7±7.9 | 38.4±5.8 |
| plGFBP-1 (ng/ml) | 68.8±9.4 | 217.1±25.6b |
| nplGFBP-1/ total IGFBP-1 (%) | 34.8±3.9 | 15.0±2.6c |
| Cord blood samples are collected at delivery and phosphoisoforms of IGFBP-1 are separated by anion exchange chromatography. Levels of IGFBP-1 in each fraction are measured with an immunoradiometric assay kit aр<0.005; bp<0.00005; cр<0.0005 compared to corresponding values in AGA fetuses | ||
References
- Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem 1978; 253 (8): 2769-76.
- Rinderknecht E, Humbel RE. Primary structure of human insulin-like growth factor II. FEBS Lett 1978; 89(2): 283-6.
- Shimasaki S, Ling N. Identification and molecular characterization of insulin-like growth factor binding proteins (IGFBP-1, -2, -3, -4, -5 and -6). Prog Growth Factor Res 1991; 3 (4): 243-66.
- Ranke MB, Elmlinger M. Functional role of insulin-like growth factor binding proteins. Horn Res 1997; 48(Suppl 4):9-15.
- Bunn RC, Fowlkes JL. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol Metab 2003; 14(4): 176-81.
- Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res 2007; 7(1): 10-8.
- Holmberg JC, Haddad S, Wünsche V, et al. An in vitro model for the study of human implantation. Am J Reprod Immunol 2012; 67(2): 169-78.
- Kabir-Salmani M, Shiokawa S, Akimoto Y, et al. Characterization of morphological and cytoskeletal changes in trophoblast cells induced by insulin-like growth factor-I. J Clin Endocrinol Metab 2002; 87(12): 5751-9.
- Kabir-Salmani M, Shiokawa S, Akimoto Y, et al. Alрhavbeta3 integrin signaling pathway is involved in insulin-like growth factor I-stimulated human extravillous trophoblast cell migration. Endocrinology 2003; 144(4): 1620-30.
- Jiang H, Xun P, Luo G, Wang Q, Cai Y, Zhang Y, Yu B. Levels of insulin-like growth factors and their receptors in placenta in relation to macrosomia. Asia Pac J Clin Nutr 2009; 18(2): 171-8.
- Iñiguez G, González CA, Argandoña F, Kakarieka E, Johnson MC, Cassorla F. Expression and protein content of IGF-I and IGF-I receptor in placentas from small, adequate and large for gestational age newborns. Horm Res Paediatr 2010; 73(5): 320-7.
- Giudice LC, Dsupin BA, Irwin JC. Steroid and peptide regulation of insulin-like growth factor-binding proteins secreted by human endometrial stromal cells is dependent on stromal differentiation. J Clin Endocrinol Metab 1992; 75(5): 1235-41.
- Lee PD, Giudice LC, Conover CA, Powell DR. Insulin-like growth factor binding protein-1: recent findings and new directions. Proc Soc Exp Biol Med 1997; 216(3): 319-57.
- Frost RA, Mazella J, Tseng L. Insulin-like growth factor binding protein-1 inhibits the mitogenic effect of insulin-like growth factors and progestins in human endometrial stromal cells. Biol Reprod 1993; 49(1):104-11.
- Iwashita M, Kobayashi M, Matsuo A, Nakayama S, МimurоT, Takeda Y, Sakamoto S. Feto-maternal interaction of IGF-I and its binding proteins in fetal growth. Early Hum Dev 1992; (1-3): 187-91.
- Caufriez A, Frankenne F, Englert Y, Golstein J, Cantraine F, Hennen G, Copinschi G. Placental growth hormone as a potential regulator of maternal IGF-I during human pregnancy. Am J Physiol 1990; 258(6 Pt 1): E1014-9.
- Wilson DM, Bennett A, Adamson GD, et al. Somatomedins in pregnancy: a cross-sectional study of insulin-like growth factors I and II and somatomedin peptide content in normal human pregnancies. J Clin Endocrinol Metab 1982; 55(5): 858-61.
- Hossenlopp P, Segovia B, Lassarrе C, Roghani M, Bredon M, Binoux M. Evidence of enzymatic degradation of insulin-like growth factor-binding proteins in the 150K complex during pregnancy. J Clin Endocrinol Metab 1990; 71(4): 797-805.
- Giudice LC, Farrеll EM, Pham H, Lamson G, Rosenfeld RG. Insulin-like growth factor binding proteins in maternal serum throughout gestation and in the puerperium: effects of a pregnancy-associated serum protease activity. J Clin Endocrinol Metab 1990; 71(4): 806-16.
- Sakai K, Iwashita M, Takeda Y. Profiles of insulin-like growth factor binding proteins and the protease activity in the maternal circulation and its local regulation between plaсenta and dеcidua. Endocr J 1997; 44(3): 409-17.
- Howell RJ, Perry LA, Choglay NS, Bohn H, Chard T. Placental protein 12 (PP12): a new test for the prediction of the small-for-gestational-age infant. Br J Obstet Gynaecol 1985; 92(11): 1141-4.
- Takeda Y, Iwashita M. Role of growth factors on fetal growth and maturation. Ann Acad Med Singapore 1993; 22(2): 134-41.
- Frauman AG, Tsuzaki S, Moses AC. The binding characteristics and biological effects in FRTL5 cells of placental protein-12, an insulin-like growth factor-binding protein purified from human amniotic fluid. Endocrinology 1989; 124(5): 2289-96.
- Burch WM, Correa J, Shively JE, Powell DR. The 25-kilodalton insulin-like growth factor (IGF)-bindling protein inhibits both basal and IGF-I-mediated growth of chick embryo pelvic сartilagе in vitro. J Clin Endocrinol Metab 1990; 70(1): 173-80.
- Ritvos O, Ranta T, Jalkanen J, Suikkari AM, Voutilainen R, Bohn H, Rutanen EM. Insulin-like growth factor (IGF) binding protein from human decidua inhibits the binding and biological action of IGF-I in cultured choriocarcinoma cells. Endocrinology 1988; 122(5): 2150-7.
- Rutanen EM, Pekonen F, Mäkinеn T. Soluble 34K binding protein inhibits the binding of insulin-like growth factor I to its cell receptors in human secretory phase endometrium: evidence for autocrine/paracrine regulation of growth factor action. J Clin Endocrinol Metab 1988; 66(1): 173-80.
- Bennett A, Wilson DM, Liu F, Nagashima R, Rosenfeld RG, Hintz RL. Levels of insulin-like growth factors I and II in human cord blood. J Clin Endocrinol Metab 1983; 57(3): 609-12.
- Wang HS, Lim J, English J, Irvine L, Chard T. The concentration of insulin-like growth factor-I and insulin-likе growth faсtоr-binding protein-1 in human umbilical cord serum at delivery: relation to fetal weight. J Endocrinol 1991; 129(3): 459-64.
- Verhaeghe J, Van Bree R, Van Herck E, Laureys J, Bouillon R, Van Assche FA. C-peptide, insulin-like growth factors I and II, and insulin-like growth factor binding protein-1 in umbilical cord serum: correlations with birth weight. Am J Obstet Gynecol 1993; 169(1): 89-97.
- Iwashita M. Physiological significance of IGF-I and its binding proteins on fetal growth and maturation. Nihon Sanka Fujinka Gakkai Zasshi 1994; 46(8): 660-72.
- Goswami R, Lacson R, Yang E, Sam R, Unterman T. Functional analysis of glucocorticoid and insulin response sequences in the rat insulin-like growth factorbinding protein-1 promoter. Endocrinology 1994; 134(2): 736-43.
- Suh DS, Ooi GT, Rechler MM. Identification of cis-elements mediating the stimulation of rat insulin-like growth factor-binding protein-1 promoter activity by dexamethasone, cyclic adenosine 3’,5’-monophosphate, and phorbol esters, and inhibition by insulin. Mol Endocrinol 1994; 8(6): 794-805.
- Takenaka A, Komori K, Morishita T, Takahashi SI, Hidaka T, Noguchi T. Amino acid regulation of gene transcription of rat insulin-like growth factor-binding protein-1. J Endocrinol 2000; 164(3): R11-6.
- Cetin I, Marconi AM, Bozzetti P, Sereni LP, Corbetta C, Pardi G, Battaglia FC. Umbilical amino acid concentrations in appropriate and small for gestational age infants: a biochemical difference present in utero. Am J Obstet Gynecol 1988; 158(1): 120-6.
- Sеferovic MD, Ali R, Kamei H, et al. Hypoxia and leucine deprivation induce human insulin-like growth factor binding protein-1 hyperphosphorylation and increase its biological activity. Endocrinology 2009; 150(1): 220-31.
- Tanaka K, Sakai K, Matsushima M, et al. Branched-chain amino acids regulate insulin-like growth factor-binding protein 1 (IGFBP1) production by decidua and influence trophoblast migration through IGFBP1. Mol Hum Reprod 2016; 22(8):890-9.
- Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr 2006; 136(Suppl 1): 227S-31S.
- Jones JI, D'Ercole AJ, Camacho-Hubner C, Clemmons DR. Phosphorylation of insulin-like growth factor (IGF)-binding protein 1 in cell culture and in vivo: effects on affinity for IGF-I. Proc Natl Acad Sci USA 1991; 88(17): 7481-5.
- Jones JI, Busbу WН Jr, Wright G, Clemmons DR. Human IGFBP-1 is phosphorylatеd on 3 serine residues: effects of site-directed mutagenesis of the major phosphoserine. Growth Regul 1993; 3(1): 37-40.
- Frost RA, Tseng L. Insulin-like growth factor-binding protein-1 is phosphorylatеd by cultured human endometrial stromal cells and multiple protein kinases in vitro. J Biol Chem 1991; 266(27): 18082-8.
- Yu J, Iwashita M, Kudo Y, Takeda Y. Phosphorуlatеd insulin-like growth factor (IGF)-binding protein-1 (IGFBP-1) inhibits while non-phosphorylated IGFBP-1 stimulates IGF-I-induced amino acid uptake by cultured trophoblast cells. Growth Horm IGF Res 1998; 8(1): 65-70.
- Iwashita M, Sakai K, Kudo Y, Takeda Y. Physiological significance of insulin-like growth factor (IGF)-binding protein-1 (IGFBP-1) phosphoisoforms in fetal growth. Clin Pediatr Endocrinol 1998; 7(Suppl 11): 31-7.
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 1995; 16(1): 3-34.
- Jyung RW, Mustoe JA, Busby WH, Clemmons DR. Increased wound-breaking strength induced by insulin-like growth factor I in combination with insulin-like growth factor binding protein-1. Surgery 1994; 115(2): 233-9.
- Mendoza AE, Maile LA, Cairns BA, Maile R. Burn injury induces high levels of phosphorylated insulin-like growth factor binding protein-1. Int J Burns Trauma 2013; 3(4): 180-9.
- Frost RA, Berеket А, Wilson TА, Wojnar MM, Lang CH, Gelato MC. Phosphorylation of insulin-like growth factor binding protein-1 in patients with insulin-dependent diabetes mellitus and severe trauma. J Clin Endocrinol Metab 1994; 78(6) 1533-5.
- Iwashita M, Sakai K, Kudo Y, Тakeda Y. Phosphoisoforms of insulin-like growth factor binding protein-1 in appropriate-for-gestational-age and small-for-gestational-age fetuses. Growth Horm IGF Res 1998; 8(6): 487-93.
Download Full Article
- Mitsutoshi Iwashita, MD: Fetal growth and insulin-like growth factor system (2007, Journal of Hungarian Obstetricians and Gynaecologists, PDF, 7 pages, English, 584KB)