LITERATURE - 2020, Journal of Hungarian Obstetricians and Gynaecologists
MicroRNAs in Endometriosis
Novel non-hormonal targets for early diagnosis

Authors: Linda S. Ross1, Martin Götte2, Ludwig Kiesel3
1Department of Obstetrics and Gynecology, St. Franziskus Hospital, Münster, Germany
2Department of Obstetrics and Gynecology, Münster University Hospital, Münster, Germany
3Corresponding author
Endometriosis is a common disease among women in reproductive age leading to debilitating pain and infertility hence depicting a serious personal as well as economic burden. Up to now the gold standard in diagnosing endometriosis continues to be invasive by laparoscopy. Non-invasive biomarkers, in particular microRNAs, are being investigated in order to find a sensitive and specific new tool to non-invasively make an early diagnosis of endometriosis. Although research findings are promising, at the present day still no biomarker has been found.
Keywords: biomarker, mikroRNS, endometriozis
Endometriosis
Endometriosis is an estrogen dependent inflammatory disease that is thought to affect up to 10% of all women between menarche and menopause. It is characterized by endometriosis lesions growing in different locations outside the uterus most commonly in the ovaries or the pelvic peritoneum, leading to cyclical and non-cyclical symptoms, i.e. pelvic pain, dysmenorrhea and infertility [1]. Owing to the variety and complexity of the presented symptoms, diagnosis is often delayed while symptoms are misinterpreted. Today laparoscopy is still the gold standard for diagnosing endometriosis hence non-invasive biomarkers are urgently needed and present a current scientific challenge.
What are mRNAs?
MicroRNAs are small, non-coding RNA molecules that play a major role in gene expression. They are transcribed and processed as precursor DNAs inside the cell nucleus comprising of 19-24 nucleotides. In the cytoplasm microRNAs bind the 3’ end of the target messenger RNAs (mRNA) and hence are able to interfere in translation or induce the deterioration of mRNAs via the “RNA-induced silencing complex” (RISC). This way microRNAs are able to influence cell migration, proliferation, angiogenesis, inflammation and apoptosis via negative regulation of gene expression (Figure 1.) [2, 3]. Among epigenetic markers microRNAs have distinguished themselves as powerful regulators of gene expression. MicroRNAs target positive regulatory motifs that are three or four proteins positively regulating each other, highly connected scaffolds and downstream network components such as signaling transcription factors or genes with promoter regions including a large number of putative transcription factor binding sites [4]. As microRNAs are tissue specific, do not undergo posttranslational modifications, are of low complexity and are stable in blood, urine or tissues they promise to potentially be useful as future biomarkers in early diagnosis of endometriosis [5]. For detection and profiling of microRNAs RT-PCR presents the gold standard due to its high accuracy and sensitivity [6]. Various studies have examined the role of mRNA in the pathogenesis of endometriosis, however they obtain varying results probably due to heterogeneity of cell types or use of varying techniques to detect microRNAs [7].
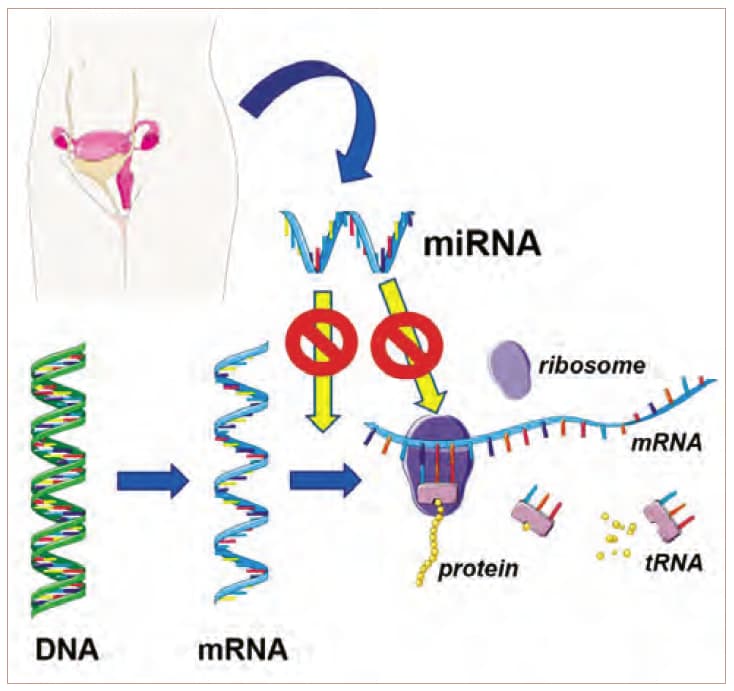
MicroRNAs can inhibit mRNA and protein expression at the posttranslational level via induction of RNA degradation in the RISC complex, of by impeding translation at the ribosome. A dysregulation of miRNAs leads to the misexpression of molecular factors that can drive the pathogenesis of endometriosis. The figure was drafted using elements of the free internet source https://smart.servier.com/
MicroRNAs and Endometriosis
40 microRNAs were found to possibly play a role in the pathogenesis of endometriosis, as they were found to be regulated differently in various studies during the past years. Agrawal and colleagues worked out the significance of these circulatory microRNAs and its role in the aethiopathogenesis of endometriosis. In the fore there are different subgroups of microRNAs (miR) that may play a pivotal role: the miR-17-5p, miR-20a, the miR-200 family, miR-199a, miR-143 and miR-145 [8].
Ohlsson-Teague and colleagues proposed a model for microRNA regulation in endometriotic lesion development. The authors put in the foreground that hypoxic cell injury and the up-regulation of hypoxia inducible transcription factors such as CREB binding protein is modified by microRNAs, in particular miR-20a and miR200b [9]. This is thought to result in increased angiogenesis, better oxygen delivery to tissues and perhaps improved survival of ectopic endometriotic lesions [10]. Secondly, pro-inflammatory conditions with elevated levels of inflammation markers leading to enhanced COX-2 transcription are described in literature [11]. COX-2 suppressor’s miR-199a and miR-16 are shown to be downregulated in endometriosis. It is hence conjectural that the upregulation of COX-2 leads to an inflammatory environment and as a result promotes neoangeogenesis, increased prostaglandin production and estradiol mediated cellular proliferation of endometriotic lesions [10]. As a number of angiogenesis-related transcripts seem to be targets of microRNAs it can be surmised that microRNAs play a significant role in regulation of angiogenesis. In the literature a variety of microRNAs are described, i.e. miR-126, enhancing VEGF and fibroblast growth factor signaling [9]. Tissue repair and TGFß-regulated pathways are central components of molecular signaling networks associated with endometriosis [12, 13]. TGFß-1 and -2 regulation is subject to miR-21 and miR-141, that are shown to be downregulated in endometriosis [9]. Ohlssen-Teague and colleagues propose the dysregulation of miR-1, miR-21, miR-141 and miR-194 may synergistically enhance TGFß signaling in endometriosis lesions by increasing TGFß expression and restraining TGIF’s suppressive activity [10]. Lastly, cell growth, proliferation and apoptosis as well as extracellular matrix remodeling are thought to be regulated by a multitude of microRNAs thus leading to enhanced survival of endometrial cells in endometriosis [10].
Placenta produces IGF-I and IGF-II and their receptors [10, 11] that stimulate trophobast migration and proliferation in an autocrine fashion. In contrast, decidual cells produce large amounts of IGFBP-1 [12] that inhibits IGF action [13].
Major microRNAs examined by current studies
Considering the subject matter from the other side of the medal one can reflect upon the major microRNAs currently or recently being examined in studies. Agrawal and colleagues from the University of Oxford published a thorough review on presently studied microRNAs. They describe a total of 40 dysregulated microRNAs among which miR-17-5p, miR-20a, miR-200, miR-199a, miR-143, and miR-145 are suggested to play a major role in the pathogenesis of endometriosis [8].
Highlighted is the miR-200 family that consists of miR-200a, miR-200b and miR-141 being dysregulated both in blood and tissue. The combination of the above named microRNAs is shown to have sensitivity and a specificity of 84.4% and 66.7% [9]. Ohlsson et al. point out that miR-200b is thought to be decreased in endometriomas compared to eutopic endometrium. The downregulation of the miR200 family may induce the epithelial-mesenchymal transition characteristic of endometriosis [14, 15]. Indeed, a dysregulation of the anti-metastatic adhesion molecule E-cadherin due to altered miR-200b expression was shown to result in altered invasive growth of endometriotic cells in vitro [15]. Next to the miR-200 family miR-20a is described as a leading biomarker for endometriosis being down-regulated in several studies. This leads to increased concentrations of TGFß and IL-8, playing an important role in inflammation and tissue repair and potentially explaining the growth of endometriotic lesions [15]. Furthermore miR-20a was found to be upregulated in patients with endometrioma leading to upregulated fibroblast growth factor-9, a potent mitogen that stimulates both endothelial and endometrial cell proliferation [17]. Several further microRNAs are described in literature as up- or downregulated, i.e. miR-199a or miR-145 thus showing controversial outcomes [18]. At the functional level, miR-145 was shown to influence invasive growth of endometriotic cells, which was due to a targeting of cytoskeletal elements [19]. Moreover, miR-145 regulated proliferation and the stem cell phenotype of endometriotic cells, as a prerequisite for unlimited growth at ectopic sites [19]. Various studies have examined the role of mRNA in the pathogenesis of endometriosis however they often obtain differing results. This limited overlap between the proposed disease-related microRNAs in endometriosis is probably due to heterogeneity of cell types or use of varying techniques to detect microRNAs [7]. Moreover, methods for data normalization are differing, as there is no broad consensus on housekeeping genes for miRNA-based studies [7]. While some miRNAs reach good values for sensitivity and specificity, these values are currently not superior to current diagnostic methods [8, 20–21]. While a single study reported excellent sensitivity and specificity values of of 95.6% and 91.4% for serum miR-122, and of 100% and 100.0% for miR-199a for the detection of endometriosis [22], these data were not in concordance with an earlier study. An overview on important studies on the diagnostic value of miRNAs in endometriosis is provided in table I.
Future perspective
Up to now no single microRNA seems to be applicable as a diagnostic marker neither has been found a combination of dysregulated microRNAs that could be used as diagnostic tool. However further progress and research on microRNAs is valuable and promising for future medicine. Circulating microRNAs could be novel targets for early diagnosis and treatment monitoring on the one hand as well as major therapeutic targets in the therapy of endometriosis. Ohlsson-Teague and colleagues suggest that in the future a non-invasive blood test that identifies microRNAs secreted from endometriotic tissue could be a precious cornerstone in the non-invasive diagnosis of endometriosis. Furthermore the authors suggest microRNAs as potential non-hormonal therapeutic targets. Antagonisms of microRNAs via anti-microRNAs, use of microRNA decoys or use of microRNA mimics in order to increase the degree of miRNA regulation and suppress transcription of mRNAs that promote disease activity are potential therapeutic strategies in future treatment of endometriosis [9].
| Table I. Diagnostic value of serum miRNAs in endometriosis | |||
|---|---|---|---|
| microRNA | Sensitivity (%) | Specificity (%) | Reference |
| miR-20a miR-22 miR-17-5p | 60 90 60 | 90 90 80 | Jia et al. 2013 [24] |
| mi-125b-5p | 100 | 96 | Cosar et al. 2016 [25] |
| let-7d (proliferative phase) | 83.3 | 100 | Cho et al. 2015 [26] |
| miR-122 miR-141-5p miR-145 miR-199a | 80 71.69 70 78.33 | 76 96 96 76 | Wang et al. 2013 [18] |
| miR-16+miR-191+miR-195 | 88 | 60 | Suryavanshi et al. 2013 [27] |
| miR-141 miR-200a miR-200b | 71.9 90.6 90.6 | 70.8 62.5 70.8 | Rekker et al. 2015 [28] |
| miR-155+miR574-3p+miR139-3p | 83 | 51 | Nisenblatt et al. 2019 [20] |
| laparoscopy | 94 | 79 | Wykes et al. 2004 [23] |
| Compilation of studies summarized in references [8, 20–21]. Values for the gold standard laparoscopy are from reference [23]. | |||
Conclusion
MicroRNAs are promising future targets for diagnosis as well as treatment of endometriosis. However, up to now no single microRNA or combination of microRNAs are found to meet the requirements as diagnostic marker. Thus, future research is promising in order to find a novel non-invasive way for early diagnosis or even for new non-hormonal treatment options of endometriosis, a debilitating and burdening disease that affects a great number of women in their reproductive years.
References
- Fuldeore MJ, Soliman AM. Prevalence and Symptomatic Burden of Diagnosed Endometriosis in the United States: National Estimates from a Cross-Sectional Survey of 59,411 Women. Gynecol Obstet Invest 2017; 82: 453–461.
- Götte M, Kiesel L. Pathophysiologie der Endometriose: Neue Erkenntnisse. Gynäkologische Endokrinol 2016; 14: 2–8.
- Neubauer C, Kiesel L, Götte M. MicroRNAs and the pathogenesis of endometriosis. J Endometr 2012; 4: 1–16.
- Cui Q, Yu Z, Purisima E, et al. Principles of microRNA regulation of a human cellular signaling network. Mol Syst Biol 2006; 2: 46.
- Weber JA, Baxter DH, Zhang S et al. The microRNA spectrum in 12 body fluids. Clin Chem 2010; 56: 1733–1741.
- D’haene B, Vandesompele J, Hellemans J. Accurate and objective copy number profiling using real-time quantitative PCR. Methods 2010; 50: 262–270.
- Saare M, Rekker K, Laisk Podar T, et al. Challenges in endometriosis miRNA studies – From tissue heterogeneity to disease specific miRNAs. Biochim Biophys Acta 2017; 1863: 2282–2292.
- Agrawal S, Tapmeier T, Rahmioglu N. The miRNA Mirage: How Close Are We to Finding a Non-Invasive Diagnostic Biomarker in Endometriosis? A Systematic Review. Int J Mol Sci 2018; 19: 599.
- Ohlsson Teague EMC, Van der Hoek KH, Van der Hoek M, et al. MicroRNAregulated pathways associated with endometriosis. Mol Endocrinol 2009; 23: 265–275.
- Teague EM, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update 2010; 16: 142–65.
- Wu MH, Sun HS, Lin CC, et al. Distinct mechanisms regulate cyclooxygenase-1 and -2 in peritoneal macrophages of women with and without endometriosis. Mol Hum Reprod 2002; 8: 1103–1110.
- Komiyama S, Aoki D, Komiyama M, Nozawa S. Local activation of TGF-beta1 at endometriosis sites. J Reprod Med 2007; 52: 306–312.
- Kyama CM, Overbergh L, Mihalyi A et al. Endometrial and peritoneal expression of aromatase, cytokines, and adhesion factors in women with endometriosis. Fertil Steril 2008; 89: 301–310.
- Korpal M, Kang Y. The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol 2008; 5: 115–119.
- Eggers JC, Martino V, Reinbold R, et al. microRNA miR-200b affects proliferation, invasiveness and stemness of endometriotic cells by targeting ZEB1, ZEB2 and KLF4. Reprod Biomed Online 2016; 32: 434–45.
- Ramón LA, Braza-Boïls A, Gilabert-Estellés J. microRNAs expression in endometriosis and their relation to angiogenic factor. Hum Reprod 2011; 26: 1082–90.
- Lin SC, Wang CC, Wu MH. Hypoxia-induced microRNA-20a expression increases ERK phosphorylation and angiogenic gene expression in endometriotic stromal cells. J Clin Endocrinol Metab 2012; 97: E1515–23.
- Wang WT, Zhao YN, Han BW. Circulating microRNAs identified in a genomewide serum microRNA expression analysis as noninvasive biomarkers for endometriosis. J Clin Endocrinol Metab 2013; 98: 281–9.
- Adammek M, Greve B, Kässens N, et al. MicroRNA miR-145 inhibits proliferation, invasiveness, and stem cell phenotype of an in vitro endometriosis model by targeting multiple cytoskeletal elements and pluripotency factors. Fertil Steril 2013; 99: 1346–1355.e5.
- Nisenblat V, Sharkey DJ, Wang Z, et al. Plasma miRNAs Display Limited Potential as Diagnostic Tools for Endometriosis. J Clin Endocrinol Metab 2019; 104: 1999–2022.
- Moga MA, Bălan A, Dimienescu OG. Circulating miRNAs as Biomarkers for Endometriosis and Endometriosis-Related Ovarian Cancer-An Overview. J Clin Med 2019; 8: 735.
- Maged AM, Deeb WS, El Amir A, et al. Diagnostic accuracy of serum miR-122 and miR-199a in women with endometriosis. Int J Gynaecol Obstet 2018; 141: 14–19.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG 2004; 111: 1204–12.
- Jia SZ, Yang Y, Lang J, et al. Plasma miR-17-5p, miR-20a and miR-22 are downregulated in women with endometriosis. Hum Reprod 2013; 28: 322–330.
- Cosar E, Mamillapalli R, Ersoy G, et al. Serum microRNAs as diagnostic markers of endometriosis: A comprehensive array-based analysis. Fertil Steril 2016; 106: 402–409.
- Cho S, Mutlu L, Grechukhina O, et al. Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril 2015; 103: 1252–1260.e1.
- Suryawanshi S, Vlad AM, Lin HM, et al. Plasma MicroRNAs as novel biomarkers for endometriosis and endometriosis-associated ovarian cancer. Clin Cancer Re 2013; 19: 1213–1224.
- Rekker K, Saare M, Roost AM, et al. Circulating miR-200-family micro-RNAs have altered plasma levels in patients with endometriosis and vary with blood collection time. Fertil Steril 2015; 104: 938–946.e2.
Download Full Article
- Linda S. Ross: MicroRNAs in Endometriosis (2020, Journal of Hungarian Obstetricians and Gynaecologists, PDF, 4 pages, English, 1.7MB)