LITERATURE - 2018, Journal of Hungarian Obstetricians and Gynaecologists
Placental location after Caesarean section

Authors: Zorić Jelena1,2, Vejnović Aleksandra1,2, Kokanov Dunja1, Subotin Divna1, Šproh Beljička Jarmila1, Đorđević Dragan1
1Faculty of Medicine, University of Novi Sad, Serbia (Brkić Snežana MD, PhD)
2Department of Gynecology and Obstetrics, Clinical Center of Vojvodina (Vejnović Tihomir MD, PhD)
Objective: Since the presence of placenta accreta is more common in the pregnancies preceded by Caesarean section (CS), and that in the majority of placenta accreta cases invasion of the anterior uterine wall is found, the assumption is evoked that CS scars are predilection sites for implantation and further development of the placenta.
Methods: Medical records of 1018 women with CS were reviewed retrospectively. Placental location was identified from preoperative ultrasonography reports and categorized as anterior and non-anterior (posterior, fundal, or lateral). The frequency of certain placental location was calculated depending on the number of previous CSs and the interval between two CSs. Birth weights of the newborn and placenta, blood loss and number of abortions were correlated depending on placental location. All data were statistically analyzed.
Results: The mean maternal age was 31.5±5.2 years. There was no significant difference in distribution of placental location depending on the number of CS. Number of abortions, blood loss and birth weight were significantly different depending on the placental location and number of CSs (p=0.037; p=0.023; p=0.01). Anterior placenta was more frequent when the interval between two CSs was shorter than 3 years (p<0.05).
Conclusions: The healing process after CS seems to extend well beyond into postpartum, and the inflammatory process allows placental implantation in the anterior wall of the uterus. After it is completed (3-years after CS according to our results), the section area becomes unsuitable for implantation. Further investigation is needed to understand the role of surgical closure technique and genetic makeup on the healing process.
Keywords: placenta, Caesarean section, scar
Introduction
Caesarean section (CS) is one of the most common surgical operations the rate of which is rising steadily. At the Clinic of Gynecology and Obstetrics in Novi Sad, the CS rate has almost tripled in the past twenty years, and today it makes up 32% of all deliveries. The rise is commonly attributed to the increasing number of conditions that lead to CS, such as maternal obesity, gestational diabetes, or multiple gestations as well as Caesarean delivery on demand. However, the previous Caesarean section appears to be the only indication in 26% of the patients in Novi Sad.
Although CS is performed routinely with rare short term complications that have to be treated surgically (intraabdominal bleeding 0.1% of all CS cases, subfascial hematoma 0.05%, uterine atony 0.5%, and wound dehiscence 0.15%, respectively), CS might have long term consequences and may cause severe adverse outcome for both the mother and the baby in future pregnancies. The most devastating complication is placenta accrete, which leads to profuse postpartum hemorrhage and peripartum hysterectomy. The reported incidence of placenta accreta cases is 3/1000 deliveries; however, the risk exponentially grows with the number of CS in a patient and presence of placenta previa [1]. According to the study of 116 placenta previa cases at the Clinic in Novi Sad, 17 placenta accreta cases were confirmed in histopathology, 82.3% of which invaded the anterior uterine wall [2].
Since the severe placental complications happen at the site of uterine scar in the majority of the cases, and they become more frequent with the higher number of previous CSs, the hypothesis is set that CS scar is the predilection site for implantation and further development of the placenta.
This study aimed to assess whether the anterior placenta is more frequent in patients with iterative Caesarean section. We also tried to evaluate whether certain placental locations influence the characteristics of the placenta, the newborn, or the blood loss.
Materials and methods
This retrospective study was conducted at the Clinic of Gynecology and Obstetrics of the Clinical Center of Vojvodina in Novi Sad, Serbia. The study protocol was approved by the hospital Scientific and Ethics Committees.
Medical records of women delivering by Caesarean section in the period between October 2014 and January 2016 were collected and reviewed. We considered patients with single fetus pregnancy to whom ultrasonography was performed one day preoperatively eligible for the study. The location of the placenta was stated as anterior or nonanterior (posterior, fundal, or lateral), regarding its relation to the uterine scar. Patients were grouped according to the number of Caesarean sections and placental location.
Patients with multiple pregnancies, abnormal placentation, and with incomplete clinical records were excluded. Other data collected from the medical records were maternal age, weight and height, birth weight and birth length, gravidity, parity, number of abortions, blood loss at delivery, and placental fresh weight.
were maternal age, weight and height, birth weight and birth length, gravidity, parity, number of abortions, blood loss at delivery, and placental fresh weight.
The chi-square test was used to calculate the frequency of certain placental locations depending on the number of previous Caesarean sections and the time passed since the previous CS. The correlation between the placental location and the newborn weight was calculated by using the ANOVA test. The same test was used for correlation between the placental location and the placental fresh weight as well as the placental location and blood loss. Placental fresh weight and newborn weight as well as weight of the mother were correlated by using the Pearson’s test.
Results
In the time interval considered, 1018 women met the inclusion criteria and were enrolled in the study. For 511/1018 (50.2%) women, this was the first CS. Overall, 507/1018 (49.8%) women underwent Caesarean section before, 417/1018 (41%) of which had one, 82/1018 (8%) had two, and 8/1018 (0.8%) women had three CSs.
The mean maternal age was 31.5±5.2 years, mean maternal weight was 81.4±14.1 kg, and the mean maternal height was 166.3±9.3 cm.
Concerning the newborn characteristics, 50.1% were female and 49.9% male, mean birth weight was 3294.8±650.8 g, and mean birth length was 49.2±3 cm. The mean gestational age at birth was 38.8±2 weeks. The mean placental fresh weight was 597.5±104 g.
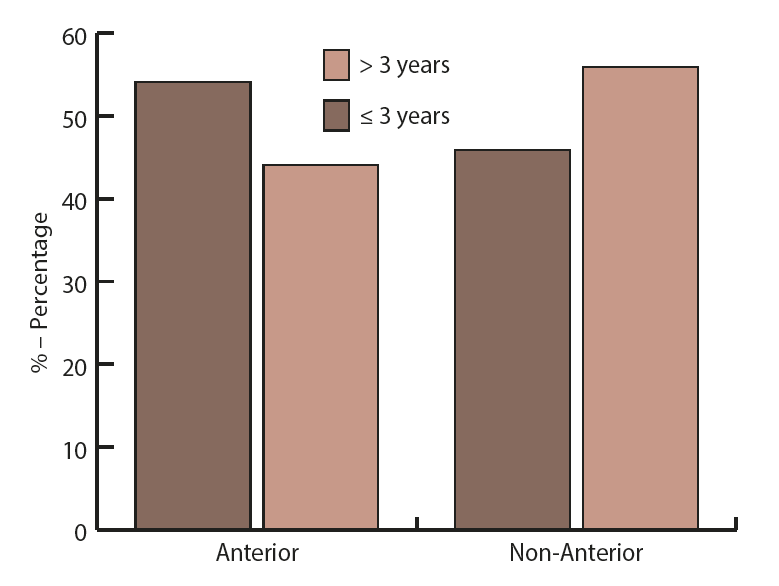
The anterior location of the placenta was less common, with an increasing number of prior CSs, and the fundal placenta showed the same trend (Figure 1.). The opposite happened to the posterior placenta, which occurred more frequently. The lateral placenta did not have a regular trend.
| Table 1. Association between the number of previous Caesarean sections and placental location | |||||||
|---|---|---|---|---|---|---|---|
| Placental location | Total | ||||||
| Anterior | Posterior | Fundal | Lateral | ||||
| Number of previous Caesarean sections | 0 | Count | 250 | 228 | 21 | 12 | 511 |
| % within CS count | 48.9% | 44.6% | 4.1% | 2.3% | 100.0% | ||
| % within Placental location | 50.6% | 48.8% | 56.8% | 60.0% | 50.2% | ||
| % of Total | 24.6% | 22.4% | 2.1% | 1.2% | 50.2% | ||
| 1 | Count | 203 | 197 | 14 | 3 | 417 | |
| % within CS count | 48.7% | 47.2% | 3.4% | 0.7% | 100.0% | ||
| % within Placental location | 41.1% | 42.2% | 37.8% | 15.0% | 41.0% | ||
| % of Total | 19.9% | 19.4% | 1.4% | 0.3% | 41.0% | ||
| 2 | Count | 35 | 40 | 2 | 5 | 82 | |
| % within CS count | 42.7% | 48.8% | 2.4% | 6.1% | 100.0% | ||
| % within Placental location | 7.1% | 8.6% | 5.4% | 25.0% | 8.1% | ||
| % of Total | 3.4% | 3.9% | 0.2% | 0.5% | 8.1% | ||
| 3 | Count | 6 | 2 | 0 | 0 | 8 | |
| % within CS count | 75.0% | 25.0% | 0.0% | 0.0% | 100.0% | ||
| % within Placental location | 1.2% | 0.4% | 0.0% | 0.0% | 0.8% | ||
| % of Total | 0.6% | 0.2% | 0.0% | 0.0% | 0.8% | ||
| Total | Count | 494 | 467 | 37 | 20 | 1018 | |
| % within CS count | 48.5% | 45.9% | 3.6% | 2.0% | 100.0% | ||
| % within Placental location | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | ||
| % of Total | 48.5% | 45.9% | 3.6% | 2.0% | 100.0% | ||
| CS – Caesarean section; % – percentage | |||||||
However, there was no significant association between the number of previous CSs and placental location (p=0.091). Since the number of women who previously underwent Caesarean 3 times was 8 (0.8%), and it was 0 with fundal and lateral placenta location; we excluded them from the calculation.
Concerning the time interval between the previous Caesarean section and the current delivery, a significant difference in placental location was observed (Figure 1.) (p=0.018). When the time from the previous CS was equal or less than 3 years, the anterior placenta occurred more often (54.1%). Contrary, when this period was more than 3 years, non-anterior placenta was found more frequently (55.9%).
Concerning newborn weight, significant correlation has been found depending on the placental location (Table 2.) (p=0.042). When only the anterior placentas were observed, a significant result was obtained. Newborns of mothers from the second CS were heavier than those from the first CS [x(CS1)=3172.6 g; x(CS2)=3389.1 g; p=0,01].
| Table 2. Association between newborn weight and placental location | |||||||
|---|---|---|---|---|---|---|---|
| N | X | SD | Min | Max | F | p | |
| Anterior | 405 | 3285.531 | 635.708 | 550 | 5030 | 2.745 | 0.042 |
| Posterior | 390 | 3297.513 | 657.309 | 770 | 4780 | ||
| Fundal | 32 | 3010.937 | 729.624 | 1440 | 4090 | ||
| Lateral | 19 | 3045.789 | 632.722 | 1580 | 4290 | ||
| Total | 846 | 3275.284 | 651.368 | 550 | 5030 | ||
| N – absolute number, X – average value, SD – standard deviation, Min – minimal value, Max – maximal value | |||||||
The highest average placental fresh weight occurred in the anterior located placenta (620.9 g), and the lowest average placental fresh weight was in fundal placenta (Table 3.), (585.2 g). Anterior placenta showed the highest average blood loss (698 mL), and the fundal placenta presented the lowest average blood loss (Table 4.), (440 mL), but no significant correlation has been found (p=0.77 and p=0.27, respectively) considering the correlation of placental fresh weight as well as blood loss with placental location.
However, comparing blood loss in anterior placentas between first and second CS, there was significantly less bleeding in the second CS [x(CS1)=655 mL; x(CS2)=503 mL; p=0.023].
Finally, patients with anterior placenta had more abortions before the second CS than before the first CS (p=0.037).
Discussion
In this study, we did not find significant association between the number of previous CSs and the placental location. Nevertheless, some regularity was been detected. The higher the number of the previous CSs, the less common anterior and fundal placentas were. The opposite happened to posterior placenta that occurred more frequently.
There is a body of published papers stating different results. Some of them claim that the presence of CS scar has no impact on placental location [3]. A recent prospective cohort study has found that the CS scar was related to an increased risk of anterior placental implantation [4]. Getting the same results as we did, Naji et al. have concluded that women with a previous history of CS are significantly placenta located on the posterior wall of the uterus [5]. A possible explanation may lie in the higher rate of spontaneous abortions when the implantation is at the site of the CS scar, i.e., the anterior site of the uterus [6].
Our study suggests the important role of the time passed from the prior CS concerning the implantation site in the subsequent pregnancy. When the time since the previous CS was equal or less than 3 years, the anterior placenta occurred more often and contrary, when this period was more than 3 years, non-anterior placenta was more often present. Lately, studies have increasingly been focusing on the uterine inflammatory response and its association with a successful implantation. It is well known that the implantation is a physiological inflammatory process characterized by an ingress of leukocytes, production of chemokines, pro-inflammatory cytokines, and other inflammatory mediators [7, 8, 9]. There is a rising interest on the potential factors that may influence this process [10]. There is a body of research confirming that a local endometrial trauma increases the implantation rate by provoking the immune system to generate an inflammatory reaction [11, 12, 13, 14, 15].
On the other hand, there is little known about the healing of the uterine scar tissue after surgical injury in women, but animal models could give us a guideline. In a mouse model, the site of the uterine scar was active and underwent significant remodeling transformation even at 15 days post- CS (7.5 months in human equivalent). In addition, several biomechanical endpoints differed between strains even 60 days post-CS (2.5 years in human equivalents) [16]. Comparing these observations with ours, after-CS healing process seems to extend well beyond into postpartum, and as a inflammatory process, it allows placental implantation in that area (i.e., in the anterior wall of the uterus). After it is completed (3 years after Caesarean section, according to our results), the section area becomes unsuitable for implantation. The information about the incidence of post CS complications in both groups, with placenta accreta on the first place would be necessary in order to make conclusions crucial for the clinicians. Further investigation is needed to make us understand the role of the surgical closure technique [2] and genetic makeup on the healing process, and the ratio of regeneration and repair in the CS scar.
| Table 3. Dependence of placental fresh weight (g) on placental location | ||||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | Standard Deviation | Standard Error | 95% Confidence Interval for Mean | Min | Max | ||
| Lower Bound | Upper Bound | |||||||
| Anterior | 453 | 620.87 | 274.328 | 12.889 | 595.54 | 646.20 | 62 | 6010 |
| Posterior | 416 | 611.82 | 89.048 | 4.366 | 603.24 | 620.40 | 68 | 1120 |
| Fundal | 29 | 585.17 | 78.904 | 14.652 | 555.16 | 615.19 | 480 | 790 |
| Lateral | 15 | 604.67 | 62.320 | 16.091 | 570.15 | 639.18 | 510 | 700 |
| Total | 913 | 615.35 | 202.999 | 6.718 | 602.16 | 628.53 | 62 | 6010 |
| Table 4. Dependence of blood loss on placental location | ||||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | Standard Deviation | Standard Error | 95% Confidence Interval for Mean | Min | Max | ||
| Lower Bound | Upper Bound | |||||||
| Anterior | 95 | 698.42 | 782.333 | 80.266 | 539.05 | 857.79 | 200 | 7000 |
| Posterior | 78 | 529.49 | 252.833 | 28.628 | 472.48 | 586.49 | 100 | 1500 |
| Fundal | 5 | 440.00 | 240.832 | 107.703 | 140.97 | 739.03 | 100 | 700 |
| Lateral | 3 | 600.00 | 173.205 | 100.000 | 169.73 | 1030.27 | 500 | 800 |
| Total | 181 | 616.85 | 596.895 | 44.367 | 529.30 | 704.40 | 100 | 700 |
Our data further showed that fetuses with fundal and lateral placenta had lower weight on delivery compared with those whose placenta was anteriorly or posteriorly located. Fung et al. have found that women with fundal and lateral placenta in second trimester had greater risk of the occurrence of small for gestational age fetuses [17]. Similar results have been reported by Kalanithi et al. showing that pregnancies complicated by IUGR are more likely than non- IUGR pregnancies to have lateral placentation [18]. Some other studies have reported no difference in newborn weight [19].
Regarding the dependence of placental fresh weight and blood loss on placental location, our study showed no significant correlation. However, the highest as well as the lowest mean placental fresh weight and blood loss were found at the same placental locations. We found the highest average blood loss in the women with anterior placentation, and this was in accordance with the recent study presenting that when the placenta is located in anterior wall there is more frequent incidence of PPH, of manual removal of placenta and prolonged duration of third stage [20]. The lowest average blood loss was within the fundal placenta. The study about myometrial thickness during human labor has shown significant thickening of the anterior and fundal myometrium during the second stage of labor, and significantly thicker anterior and posterior walls after the completion of the third stage of labor; thus, it may represent the dominant role of certain walls in these two stages of labor [21]. Adding the well-known fact that progesterone from the placenta blocks myometrial contractility primarily at the site of implantation, we could search for explanation of the different amount of blood loss in the wall function and myometrial contractility. A prospective study has showed that the length of the third stage of labor is shorter if the placenta is located at the fundus, which is in accordance with the previous hypothesis [22].
Still, very similar results regarding neonatal birth weight, placental fresh weight and blood loss depending on the placental location may point to a better vascularization of certain uterine walls. Further studies need to be conducted in order to find the answer.
The authors declare no conflicts of interest.
References
- Placenta accreta. Committee Opinion No. 529. American College of Obstetricians and Gynecologists. Obstet Gynecol 2012; 120: 207–11.
- Vejnović T, Vejnović A. New technique in obstetrics: Vejnović modification of caesarean section. Is there an impact on the frequency of placenta increta/percreta? Jatros Medizin für die Frau 3/16. p. 26–9. Available from: http://ch.universimed.com/files/ grafik/Zeitungen_2016/Frau_1603/e-papers/index.html#26/z.
- Hisley JC, Magnum C. Placental location in pregnancies following Caesarean section. JCU 1982 November; 10(9): 427–8.
- Pirjani R, Seifmanesh F, Tehranian A, Hosseini L, Heidari R, Ghajar A. et al. Placental implantation and migration following a previous caesarean section scar. ANZJOG 2017; 57: 115–7.
- Naji O, Daemen A, Smith A, Abdallah Y, Bradburn E, Giggens R, et al. Does the presence of a Caesarean section scar influence the site of placental implantation and subsequent migration in future pregnancies: a prospective case- control study. Ultrasound Obstet Gynecol 2012; 40: 557–61.
- Naji O, Wynants L, Smith A, Abdallah Y, Saso S, Stalder C, et al. Does the presence of a Caesarean section scar affect implantation site and early pregnancy outcome in women attending an early pregnancy assessment unit? Human Reproduction 2013; 28(6): 1489–96.
- Chard T. Cytokines in implantation. Hum Reprod Update 1995; 1(4): 385–96.
- Rice A, Chard T. Cytokines in implantation. Cytokine Growth Factor Rev 1998; 9(3–4): 287–96.
- Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol 1999; 181(6): 1530–6.
- Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion and preterm birth after in vitro fertilization. Fertility and sterility 2004; 82(4): 799–804.
- Zhou L, Li R, Wang R, Huang HX, Zhong K. Local injury to the endometrium in controlled ovarian hyperstimulation cycles improves implantation rates. Fertility and Sterility 2008; 89(5): 1166–76.
- Karimzadeh AM, Rozbahani MA, Tabibnejad N. Endometrial local injury improves the pregnancy rate among recurrent implantation failure patients undergoing in vitro fertilisation/intra cytoplasmic sperm injection: A randomised clinical trial. ANZJOG 2009; 49(6): 677–80.
- Barash A, Dekel N, Fieldust Sh, Segal I, Schlechtman E, Granot I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertility and Sterility 2003; 79(6): 1317–22.
- Shohayeb A, El-Khayat W. Does a single endometrial biopsy regimen (S-EBR) improve ICSI outcome in patients with repeated implantation failure? A randomised controlled trial. EJOG 2012; 164(2): 176–9.
- Gnainsky Y, Granot I, Aldo PB, Barash A, Or Yuval, Schlechtman E, et al. Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertility and Sterility 2010; 94(6): 2030–6.
- Buhimschi CS, Zhao G, Sora N, Madri JA, Buhimschi IA. Myometrial wound healing post- Caesarean delivery in the MRL/MpJ mouse model of uterine scarring. Am J Pathol 2010; 177(1): 197–207.
- Fung TY, Sahota DS, Lau TK, Leung TY, Chan LW, Chung TK. Placental site in the second trimester of pregnancy and its association with subsequent obstetric outcome. Prenat Diagn 2011; 31(6): 548–54.
- Kalanithi LE, Illuzi JL, Nossov VB, Frisbeak Y, Abdel-Razeq S, Copel J, et al. Intrauterine growth restriction and placental location. J Utrasound Med 2007; 26(11): 1481–9.
- Devarajan K, Kives S, Ray JG. Placental location and newborn weight. J Obstet Gynaecol Can 2012; 34(4): 325–9.
- Torricelli M, Vannuccini S, Moncini I, Cannoni A, Voltolini C, Conti N, et al. Anterior placental location influences onset and progress of labor and postpartum outcome. Placenta 2015; 36(4): 463–6.
- Buhimschi CS, Buhimschi IA, Malinow AM, Weiner CP. Myometrial thickness during human labor and immediately postpartum. Am J Obstet Gynecol 2003; 188(2): 553–9.
- Altay MM, IIhan AK, Haberal A. Length of the third stage of labor at term pregnancies is shorter if placenta is located at fundus: prospective study. J Obstet Gynaecol Res 2007; 33(5): 641–4.
Download Full Article
- Zorić Jelena: Placental location after Caesarean section (2018, Journal of Hungarian Obstetricians and Gynaecologists, PDF, 5 pages, English, 1.6MB)