LITERATURE - 2016, Journal of Hungarian Obstetricians and Gynaecologists
Sepsis in pregnancy

Authors: Savvas Argyridis, Spyridon Bekas, Sabaratnam Arulkumaran
St. George’s – University of Nicosia Medical School Nicosia, Cyprus
Globally sepsis is a major contributor to maternal deaths. Early advice to wash hands before attending to a pregnant mother by Semmelweis a Hungarian Physician who practiced in Austria showed a rapid decline in maternal mortality. This practice has led to less number of maternal infection but is still present especially in cases with obstetric interventions or with pre-labour rupture of membranes. This chapter describes the better understanding of the progression of localized infection to systemic spread with symptoms and signs which is sepsis to severe sepsis with organ dysfunction or tissue hypo-perfusion to septic shock with drop or blood pressure and tachycardia that may lead to organ failure needing support. The pregnant mothers are more prone to escalation from sepsis to septic shock hence the need for vigilance and detect the stages of the disease early based on symptoms and signs and initiate appropriate investigations and treatment. Early and aggressive multiple broad spectrum antibiotic and fluid therapy should be commenced after blood cultures and appropriate swab and specimen collection to identify the pathogen. Any septic focus should be removed to make antibiotic therapy to be more effective. The results of the culture and sensitivity tests would guide focused antibiotic therapy. Lactate even from capillary blood provides useful information as to the woman is in moderate or severe shock. Multidisciplinary appropriate care in an intensive care setting would provide the best care for the patient.
Keywords: sepsis, severe sepsis, septic shock, organ dysfunction, maternal death, hypoperfusion, antibiotics
Introduction
Sepsis is an important direct cause of maternal death and is on the rise and was the leading cause in the UK in some of the triennial reports [1]. This rise is believed to be related to substandard care due to failure of early recognition of symptoms and signs of sepsis and delay in initiating management [2]. Sepsis is recognized when systemic signs develop following local infection, while severe sepsis is the diagnosis when organ dysfunction or tissue hypoperfusion is detected. Septic shock is persistent tissue hypoperfusion despite adequate fluid administration which may lead to multi-organ failure [3]. According to the World Health Organization (WHO), maternal sepsis is a life-threatening condition defined as organ dysfunction due to infection in pregnancy, childbirth, post-abortion or postpartum period [4].
Systemic inflammatory response syndrome (SIRS) describes the inflammatory process that is generated by infections and is defined by the presence of two or more of the following:
- temperature >38 or <36 °C,
- heart rate >110 bpm,
- respiratory rate >24/min, PaCO2 <32 mmHg,
- white blood cell count >12000/mm3 or <4000/mm3 or >10% immature forms [5].
The commonest organisms isolated in pregnant women with sepsis include beta-haemolytic group A Streptococcus and Escherichia Coli, while mixed infections with Grampositive and Gram-negative organisms, Staphylococcus aureus, Citrobacter and Fusobacterium are also common, especially in the presence of prolonged rupture of membranes including group B Streptococcus [2]. Table 1 provides the causes of sepsis and probable infective organisms.
| Table 1. Etiologies of sepsis and probable organisms [7] | |
|---|---|
| Obstetric | Causative Organism |
| Chorioamnionitis | Streptococcus pyogenes |
| Endometritis | Streptococcus pyogenes |
| Necrotizing myometritis | Streptococcus pyogenes |
| Necrotizing Vulvitis | Streptococcus pyogenes / Staphylococcus aureus / Clostridium perfringens |
| Septic Abortion | Streptococcus/Escherichia coli / Staphylococus |
| Non-Obstetric | |
| Pyelonephritis | Escherichia coli / Klebsiella / Group B Streptococci |
| Necrotizing Fasciitis | Streptococcus pyogenes / Staphylococcus aureus / Clostridium perfringens |
| Cellulitis | Streptococcus pyogenes |
| Pharyngitis | Streptococcus pyogenes |
| Bacterial Pneumonia | Streptococcus pneumoniae / Mycoplasma pneumoniae |
| Viral Pneumonia | Influenza A and B |
| Acute appendicitis | |
| Acute Cholecystitis | |
| Gastroenteritis | |
| Mastitis | |
Risk factors
Several risk factors for sepsis have been identified, based on Confidential Enquiries into Maternal Deaths [1]. In many cases more than one factor were present. Infections in pregnancy and puerperium that can lead to sepsis include vaginal infection, urinary tract infection (acute pyelonephritis), pelvic infection (abscess, acute appendicitis), abdominal infection (acute cholecystitis, necrotizing pancreatitis, bowel infarction), pneumonia, chorioamnionitis (prolonged rupture of membranes, cervical cerclage, invasive procedures), septic abortion, placental remnants or retention (placenta accrete), necrotizing fasciitis following episiotomy or caesarean section incision, spinal abscess following regional anaesthesia and severe mastitis [6].
Maternal factors that contribute include immunosuppressive status (HIV, corticosteroid user), obesity, insulin dependent diabetes and moderate anaemia (9 g/dl).
Clinical signs and symptoms
Signs and symptoms of sepsis in pregnancy may be less distinctive than in non-pregnant women. But disease progression may be more rapid and hence prompt identification and diagnosis is required. The presentation is affected by changes in maternal hemodynamic, respiratory and renal function as well as intrapartum blood loss, use of fluids, medications, analgesia administered and route of delivery. Fever is the commonest presenting symptom, with or without chills, while other symptoms include hypothermia, tachycardia, tachypnea, hypoxia, hypotension, oliguria, impaired consciousness, diarrhoea, vomiting, cough, rash (maculopapular or purpura fulminant), pelvic pain, foul-smelling vaginal discharge, and urinary tract symptoms [7].
Complications
Maternal
Serious acute maternal morbidity following severe sepsis has been estimated at 0.4-0.6 per 1000 deliveries and it includes admission to intensive care unit, cerebral ischemia, pulmonary oedema, adult respiratory distress syndrome, myocardial infarction, acute renal failure, multiple organ failure, disseminated intravascular coagulation and death [6]. Maternal mortality rate following sepsis has been estimated at 12% of patients admitted to ICU and at 20- 28% of patients with septic shock [8].
Several prognostic factors of poor outcome have been proposed, including delayed diagnosis, depressed cardiac output, multiple organ dysfunction, high serum lactate (>4 mmol/l), and poor response to fluid resuscitation [6].
Foetal, neonatal
The foetus can be affected either directly by infection or by effects of maternal treatment (antibiotics, etc.). There is an increased risk of neonatal encephalopathy with foetal exposure to intra-amniotic inflammation, as reported by studies that found a strong association between presence of funisitis, increased interleukins 6 (IL-6), 8 (IL-8) and white cell count in amniotic fluid of foetuses that developed cerebral palsy (CP) by age 3 compared to fetuses without CP. Periventricular leukomalacia that leads to cerebral palsy is also strongly associated with intra-amniotic inflammatory factors. The presence of these factors is a strong and independent risk factor for development of cerebral palsy by age 3 [9, 10] (Table 2).
| Table 2. Complications following sepsis [6] | |
|---|---|
| Maternal | Foetal/Neonatal |
| Preterm delivery | |
| Multiple organ failure (renal, liver) | Foetal Infection/Neonatal sepsis |
| Cesarean Delivery | Respiratory Distress Syndrome |
| Cerebral / Myocardial ischemia | |
| Disseminated intravascular coagulation | ICU admission |
| Maternal Death | foetal/Neonatal Death |
Investigation
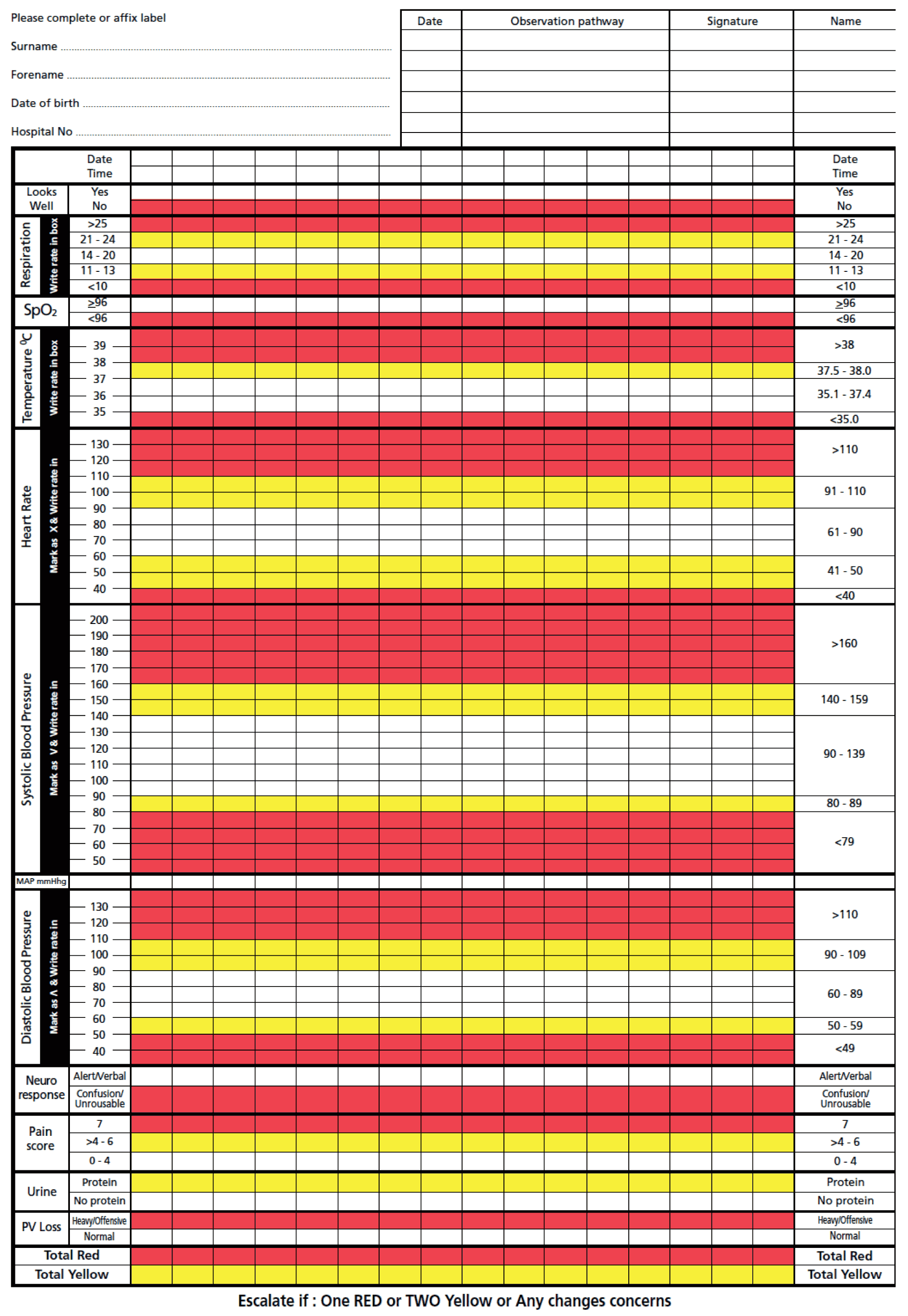
When there is a suspicion of sepsis the first step is to obtain blood cultures. Swabs from appropriate sites (high vaginal, nose) and fluid cultures (mid-stream urine, cerebrospinal fluid) should be taken prior to antibiotic administration. The regime can be changed if the results of these tests show the organisms isolated may be better treated with different antibiotics. The culture results assist to optimize antibiotic treatment. Imaging may be required to identify the site of the sepsis and to plan management. Chest x-ray, pelvic scan and abdominal MRI are imaging modalities of choice during pregnancy. Laboratory examinations required include blood glucose, electrolytes, creatinine, liver transaminases, total bilirubin, white cell count, hemoglobin, platelets, C-reactive protein (CRP), procalcitonin, international normalized ratio (INR), activated partial thromboplastin time (APTT) and lactate. Temperature, blood pressure, pulse, respiratory rate, urine output, arterial blood gases and central venous pressure are useful parameters that require close monitoring if sepsis is suspected and/or is confirmed. Use of the Modified Early Obstetric Warning Score (MEOWS) (Figure 1) chart can promptly identify seriously ill pregnant women and activate local protocols and enhance prompt care of such cases.
Diagnostic criteria
Diagnostic criteria for sepsis in pregnancy include several variables, as listed by Levy et al., with modifications by considering pregnancy-related parameters [5]. General variables include fever (temperature >38 °C), hypothermia (<36 °C), tachycardia (>100–110 bpm), tachypnoea (>20 bpm), impaired mental status, oedema and hyperglycemia (plasma glucose >7.7 mmol/l) in the absence of diabetes (Table 3).
| Table 3. Diagnostic criteria of sepsis [6] | |
|---|---|
| Clinical Signs | Laboratory Findings |
| Pyrexia (>38 °C) | Leucocytosis (>15,000/mm3) |
| Hypothermia (advanced sepsis) (<36 °C) | Leukopenia (advanced sepsis) <4,000/mm3 |
| Tachycardia (>110 beats/min) | Neutropenia (advanced sepsis) |
| Tachypnoea (>24/min) | Serum creatinine (>1.0 mg/dl) |
| Hypoxia (SaO2 <96% in air) | Serum lactate (>4.0 mmol/l) |
| Hypotension (Systolic BP <90 mmHg) | foetal/Neonatal Death |
| Oliguria | |
| Decreased level of consciousness | |
| Failure to respond to treatment | |
| Pain/Tenderness according to the aetiology | |
| Rash | |
| Clammy/molted | |
| Diarrhoea/vomiting | |
The inflammatory variables include leucocytosis (WBC >12×109/l), leukopenia (WBC <4X109/l), normocytosis with >10% immature forms, and C-reactive protein (CRP >7 mg/dl).
Leucocytosis is the commonest laboratory abnormality in septic shock during pregnancy, while in advanced sepsis leukopenia and neutropenia are developed due to bone marrow suppression.
Haemodynamic variables include hypotension (systolic blood pressure <90 mmHg, mean arterial pressure <70 mmHg). Tissue perfusion variables include serum lactate >4 mmol/l and decreased capillary refill.
Serum lactate above 4mmol/l has a strong correlation to tissue hypoxia, anaerobic metabolism and increased mortality independent of the presence or absence of multiple organ failure [11].
Organ dysfunction variables include arterial hypoxaemia (PaO2 <40 kPa), oliguria (urine output, 0.5 ml/kg for 2 hours despite fluid resuscitation), serum creatinine increase (>44.2 μmol/l), prolonged bleeding parameters (INR >1.5, APTT >60 sec), thrombocytopenia (platelet count <100×109/l), hyperbilirubinemia (serum total bilirubin >70 μmol/l) and ileus.
Staphylococcal infections have some distinct features such as diffuse macular erythroderma, desquamation (especially in palms and soles), mucous membranes hyperaemia (vaginal, oro-pharyngeal, conjunctival), myalgia and elevation of serum creatinine phosphokinase, in addition to the general signs and symptoms of sepsis.
Streptococcal infections also have some distinct features such as hypotension with abdominal pain, generalized erythematous macular rash, desquamation, soft tissue necrosis (fasciitis, myositis and gangrene), acute respiratory distress syndrome, elevated transaminases and isolation of beta-haemolytic group A Streptococcus from blood, peritoneal fluid, tissue, throat, vagina or sputum [12].
MANAGEMENT
Management of the mother
Early recognition of sepsis-related symptoms and initiation of interventions that are timely and standardized, can improve outcome in terms of mortality, morbidity and hospital stay[13] (Table 4).
| Tabe 4. Management in different phases of sepsis (6) | |
|---|---|
| Initial Phase | Maintenance Phase |
| Obtain Blood Cultures | Foetal Heart Rate monitoring |
| Broad-spectrum antibiotics (within 1 hour) | Narrow spectrum antibiotics |
| Laboratory Tests (lactate, creatinine, WBC, CRP) | Ventilation in ARDS |
| Fluid Resuscitation (maintain MAP >65 mmHg) | Stress ulcer prophylaxis (Famotidine) |
| Central Venus Pressure > 8mmHg | Thromboembolic prophylaxis |
| Vasopressors (if MAP<65 mmHg) | Corticosteroids (Hydrocortisone) |
| Oxygen Therapy | Insulin (if glucose>180 mg/dl) |
| Packed red blood cells transfusion (if Hb <7 g/dl) | |
| Search and Eliminate Source of Sepsis | |
| Intravenous Immunoglobulin (IVIG) | |
| Rash | |
| Clammy/molted | |
| Diarrhoea/vomiting | |
The initial resuscitation phase of septic shock includes oxygenation (supplemental oxygen, intubation if needed) with pulse oximetry and arterial blood gas determination. Intravenous access for fluid resuscitation and antibiotic administration is required, while blood and site-specific cultures must be taken. The initial laboratory evaluation should include a full blood count, C-reactive protein, liver function tests, kidney function tests, electrolytes, serum lactate and coagulation studies
Fluid resuscitation therapy consists of rapid infusion in order to achieve 20 ml/kg within the first hour of treatment, while subsequent fluid administration is guided by vital signs (MAP >65 mmHg), pulse oximetry, central venous monitoring (central venous pressure 8-12 mmHg) and urine output (>20-25 ml/h). Crystalloids and colloids may be used but avoidance of excessive 0.9% normal saline or Lactated Ringer’s administration is required in order to avoid pulmonary oedema due to overload [14]. Vasopressors are used when fluid resuscitation fails to resolve tissue hypoperfusion or hypotension and include norepinephrine that causes vasoconstriction and improves maternal hemodynamics and oxygen delivery substantially greater when compared to dopamine [15, 16].
Intravenous broad-spectrum antibiotics are recommended to be administered within 1 hour of suspicion of severe sepsis with or without septic shock signs [3]. A study showed that for every hour of delay to administer antibiotics, in the first 6 hours after hypotension, the survival rate dropped by 7.6% [17]. Antimicrobials that are active against Gram-negative and Gram-positive bacteria and anaerobes should be used, as infections tend to be polymicrobial. Treatment could be more targeted once culture results are available.
Cefuroxime and Cefotaxime cover Gram-positive such as Staphylococcus aureus as well as Group A and B Streptococcus, Gram-negative such as coliform but not pseudomonas nor MRSA or anaerobes. Metronidazole covers only anaerobes such as Clostridia, Bacteroides and Peptostreptococci. Ampicillin covers anaerobes, Gram-positive (Group A and Group B Streptococcus) and Gram-negative (coliform) partially while Co-Amoxyclav covers anaerobes, Gram-positive (Staphylococcus aureus, Streptococcus) but not MRSA and Gram negative (coliform). Vancomycin is suitable for anaerobes and Gram-positive including MRSA as is clindamycin which also provides some coverage for MRSA. Gentamycin is suitable for Grampositive organisms including MRSA and Gram-negative (coliform, pseudomonas), while imipenem covers Gramnegative (coliform, pseudomonas) and Gram-positive organisms excluding MRSA. Erythromycin is suitable for anaerobes and Gram-positive excluding MRSA.
Anti-infective treatment in severe sepsis includes broad spectrum antibiotics and intravenous immunoglobulin especially in Streptococcal and Staphylococcal infections as it neutralizes the effects of exotoxins and tumour necrosis factor. Administration of Clindamycin cause suppression of bacterial production of exotoxins.
Identification and elimination of the source of the sepsis is the next step after initial resuscitation and stabilization. Resection of inflammatory tissues such as in case of necrotizing fasciitis or vulvitis, drainage of a pelvic abscess or evacuating the uterine cavity from products of conception, are required as the condition will continue to deteriorate unless the source of inflammation is eliminated [18].
A multidisciplinary team should be involved in care of pregnant women in septic shock and transfer to an intensive care facility is recommended if there are signs of organ dysfunction such as hypotension, increase in serum lactate despite fluid resuscitation, acidosis, hypothermia, pulmonary oedema, need for mechanical ventilation, renal dialysis and/or decreased conscious level19. According to the ACOG and the SMFM, a critically ill pregnant woman should be cared for in a Level IV facility, where there is availability of care by maternal-fetal medicine, midwifery, medical and surgical experts with ICU and NICU facilities onsite [20].
During the maintenance phase of management, glucose control is required, and insulin is initiated in serum glucose levels of 180 mg/dl or more, as hypoglycaemia is linked to increased mortality rate [21]. Corticosteroid administration is believed to increase survival rate in septic shock patients, refractory to fluid resuscitation and vasopressor administration. It is suggested that hydrocortisone administration until shock is resolved may improve outcome [22]. Blood products transfusion is recommended in case the haemoglobin is less than 7 g/dl, active bleeding, tissue hypoxia or significant cardiac disease [23]. Enteral feeding is advised unless contraindicated, as it improves immune function and bowel blood flow [24].
Thromboembolic prophylaxis with compression devices and low molecular weight heparin helps to avoid thrombiembolism.
Foetal management
Foetal tachycardia, minimal variability and absent accelerations are cardiotocographic findings in maternal septic shock. Uterine contractions may develop as a result of inflammation and endotoxin release.
Tocolytics are contra-indicated as they have side effects (hypotension, tachycardia) that may have a deteriorating effect on the maternal status. The best option for the foetus is early delivery. Antenatal corticosteroids for lung maturation may be considered in anticipation of preterm birth due to maternal sepsis and possible foetal compromise. A transient effect of corticosteroids on white cell count is observed, with a peak within 24 hours of administration, but duration and magnitude of this effect remain uncertain [25]. Betamethasone is shown to increase white cell count in women with preterm ruptured membranes but not dexamethasone, while there was any effect on other maternal serum indicators of infection [26]. The WHO does not recommend initiation or continuation of a corticosteroid course in the setting of maternal sepsis, as it delays timely delivery of the fetus to prevent neurological insult and may exacerbate maternal infection[27, 28].
Decision for delivery is challenging and depends on maternal status and gestational age but maternal stabilization should always precede delivery. Indications for delivery include chorioamnionitis, organ failure (hepatic, renal), cardiopulmonary arrest, intrauterine death or gestational age that is associated with low morbidity or mortality (Barton and Sibai, 2012).
Prevention
According to the WHO, several measures can be adopted in order to avoid peripartum infections including digital vaginal examinations at 4-hour intervals during the first stage of labour, intrapartum antibiotic prophylaxis to women colonized with group B Streptococcus, antibiotic administration to women with prelabour rupture of membranes, manual placental removal or perineal tears as well as women undergoing caesarean section (elective or emergency). A single dose of intravenous cephalosporin administered at least 30-60 minutes prior to incision, has shown to decrease surgical site infections [29]. Hair removal around the site by clipping and not shaving prior to incision and antiseptic skin preparation is also effective. Treatment of chorioamnionitis should include ampicillin and gentamycin, while treatment of endometritis should include clindamycin and gentamycin [28]. Special care is advised in obese women as they require higher and prolonged doses of antibiotics due to decreased drug availability, increased risk of wound breakdown, hematoma formation and poor healing due to diabetes or poor hygiene.
Conclusion
Sepsis in pregnancy is a leading cause of maternal death. It is frequently related to substandard care. Prompt recognition of signs and symptoms and immediate implementation of management protocols have shown to decrease maternal morbidity and mortality. Implementation of early warning charts and investigation protocols for suspected sepsis, prompt administration of broad-spectrum antibiotics and fluid resuscitation therapy are measures that should be adopted by all maternity units. Establishment of diagnostic criteria as well as laboratory and hemodynamic parameters for maternal sepsis and shock are required in order to better standardize care in this population.
References
- Centre for Maternal and Child Enquiries (CMACE). Saving Mother’s Lives: reviewing maternal deaths to make motherhood safer: 2006–2008. BJOG 2011; 118(suppl 1): 1–203.
- Lewis G, ed. Saving Mother’s Lives: reviewing maternal deaths to make motherhood safer–2003–2005. The Seventh Report on Confidential enquiries into Maternal Deaths in the United Kingdom. London: RCOG Press; 2007.
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock. Crit Care Med 2008; 36: 296–327.
- World Health Organization. Statement on maternal sepsis. 2017. (https://www.who.int/sepsis) last accessed 25/07/2019.
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. The 2001 SCCM/ESICM/ ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31: 1250–6.
- Barton JR, Sibai BM. Management of severe sepsis and septic shock. In: Sibai BM, editor. Management of acute obstetric emergencies. 1ed Philadelphia (PA): Saunders, Elsevier pp 93–100. 2011.
- Royal College of Obstetricians and Gynaecologists (RCOG) Green-Top Guideline No 64a. Bacterial Sepsis in Pregnancy. 2012. https://www.rcog.org.uk/ en/guidelines-research-services/ guidelines/gtg64a/
- Mabie WC, Barton JR, Sibai B. Septic shock in pregnancy. Obstet Gynecol 1997; 90: 553–61.
- Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH et al. Fetal exposure to an intraamniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000; 182: 675–81.
- Yoon BH, Park CW, Chaimorapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG 2003;110(Suppl 20): 124–7.
- Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 2009; 37: 1670–7.
- Stevens DL. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis and new concepts in treatment. Emerg Infect Dis 1995; 1: 69–78.
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345: 1368–77.
- Choi PT, Yip G, Quinonez LG, Cook DJ. Crystalloids vs colloids in fluid resuscitation: a systematic review. Crit Care Med 1999; 27: 200–10.
- Martin C, Papazian L, Perrin G, Saux P, Gouin F. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock. Chest 1993; 103: 1826–31.
- DeBacker D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med 2003; 31: 1659–67.
- Kumar A, Roberts D, Wood KE, Light B, Parillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34: 1589–96.
- De Moya MA, del Carmen MG, Allain RM, Hirschberg RE, Shepard JO, Kradin RL. Case 33–2009. A 35-year old woman with fever, abdominal pain and hypotension after caesarean section. N Engl J Med 2009; 361: 1689–97.
- Plaat F, Wray S. Role of the anaesthetist in obstetric critical care. Best Pract Res Clin Obstet Gynaecol 2008; 22: 917–35.
- Menard K, Kilpatrick S, Saade G, et al. Levels of maternal care. ACOG/SMFM Consensus. Obstet Gynecol 2015; 125: 502–15.
- NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360: 1283–97.
- Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisones on mortality in patients with septic shock. JAMA 2002; 288: 862–71.
- Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion in critical care. N Engl J Med 1999; 340: 409–17.
- Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, et al. ESPEN Guidelines on Parenteral Nutrition: intensive care. Clin Nutr 2009; 28: 387–400.
- Bauer ME, Price LK, MacEachern MP, et al. Maternal leukocytosis after antenatal corticosteroid administration: a systematic review and meta-analysis. J Obstet Gynaecol 2018; 38(2): 210–216.
- Danesh A, Janghorhani M, Khalatbari S. Effects of antenatal corticosteroids on maternal serum indicators of infection in women at risk for preterm delivery: a randomized trial comparing betamethasone and dexamethasone. J Res Med Sci 2012; 17(10): 911–917.
- WHO recommendation on antenatal corticosteroid therapy in women with chorioamnionitis at risk of preterm birth. 2015 (https://www.who.int/who.int/ rhl/topics/preconception- pregnancy-childbirth-and-postpartum-care) last accessed 25/06/2019
- WHO recommendations for prevention and treatment of maternal peripartum infections. 2015. (https://www.who.int/who.int/ rhl/topics/preconception-pregnancy-childbirth- and-postpartum-care) last accessed 25/06/2019.
- Kaimal AJ, Klatnik MG, Cheng YW, Thiet MP, Connatty E, Creedy P, et al. Effect of a change in policy regarding the timing of prophylactic antibiotics on the rate of postcesarean delivery surgical-site infections. Am J Obstet Gynecol 2008; 199: 310.e1–5.
Download Full Article
- Savvas Argyridis: Sepsis in pregnancy (2016, Journal of Hungarian Obstetricians and Gynaecologists, PDF, 6 pages, English, 762KB)